2016 KCSE Chemistry Paper 1 Past Paper
Kenya certificate of Secondary Education
2016 Chemistry paper 1
1. A student investigated the effect of an electric current by passing it through some substances.
The student used inert electrodes and connected a bulb to the circuit.
The table below shows the substances used and their states.

Experiment Substance State 1 Potassium carbonate Solid 2 Copper (H) sulphate Solution 3 Sugar Solution 4 Lead (II) iodide Molten
(a) In which experiment did the bulb not light? (1 mark)
(b) Explain your answer in (a) above. (2 marks)
2. An alkanol has the following composition by mass: hydrogen 13.5%, oxygen 21.6% and carbon 64.9%.
(a) Determine the empirical formula of the alkanol. (C = 12.0, H = 1.0, 0 = 16) (2 marks)
(b) Given that the empirical formula and the molecular formula of the alkanol are the same, draw the structure of the alkanol. (1 mark)
3. The figure below shows an energy cycle.

(a) Give the name of the enthalpy change AI-11. (I mark)
(b) Determine the value of H3. (2 marks)
4. The set up below was used to investigate the reaction between dry hydrogen gas and copper (11) oxide.
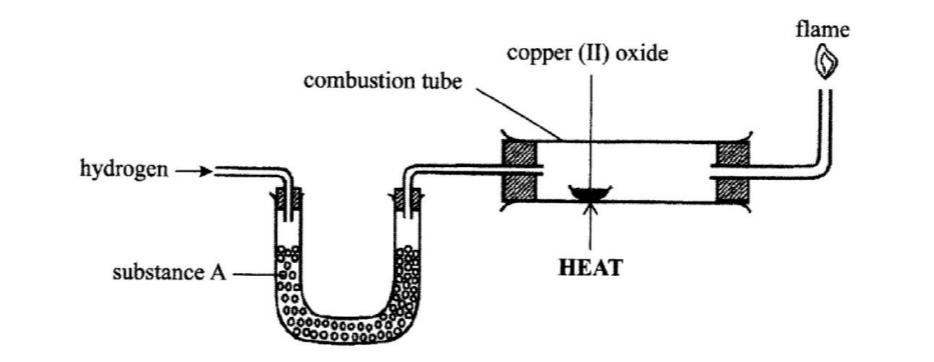
(a) Name substance A. (1 mark)
(b) State the observation made in the combustion tube. (1 mark)
(c) Explain the observation made in (b) above. (1 mark)
5. Starting with sodium metal, describe how a sample of crystals of sodium hydrogen carbonate may be prepared. (3 marks)
6. Ammonium Ion has the following structure.
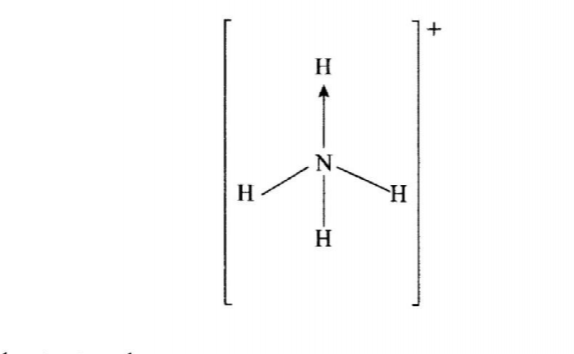
Label on the structure the:
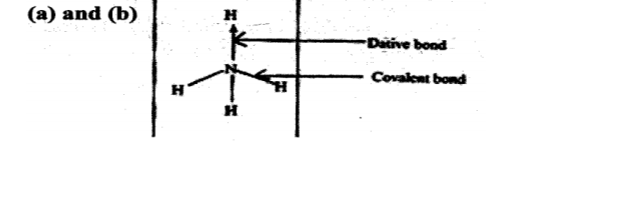
(a) Covalent bond (1 mark)
(b) Coordinate (dative) bond (1 mark)
7. When 8.53 g of sodium nitrate was heated in an open test tube, the mass of oxygen gas produced was 0.83 g.
Given the equation of the reaction as:
2NaNO3(s) -> 2NaNO2(s) + 02(g)
Calculate the percentage of sodium nitrate that was converted to sodium nitrite (Na = 23.0, N = 14.0, 0 = 16.0) (3 marks)
8. Aluminium is both malleable and ductile; (a) What is meant by?
(i) Malleable (1/2 mark)
(ii) Ductile(1/2 mark)
(i) Malleability (1/2). mark)
(ii) Ductility (1/2 mark)
9. The diagram below represents the set up that was used to prepare and collect hydrogen chloride gas in the laboratory.
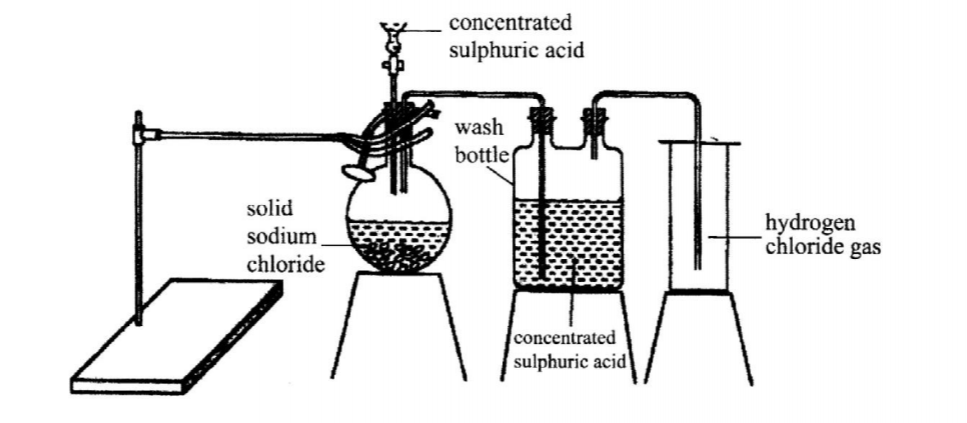
(a) State the purpose of concentrated sulphuric acid in the wash bottle. (1 mark)
(b) Write an equation for the reaction between dry hydrogen chloride gas and heated iron. ( I mark)
10. Iron (111) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (111) oxide can be obtained. (3 marks)
11. Complete the nuclear equation below. (a)
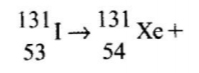
(b) The half life of
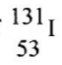
is 8 days. Determine the mass of
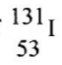
remaining if 50 grammes 50g decayed for 40 days. (2 marks) (c) Give one harmful effect of radio isotope (1 marks)
12. During an experiment, chlorine gas was bubbled into a solution of potassium iodide. (a) State the observations made. ( 1 mark)
(b) Using an ionic equation, explain why the reaction is redox. (2 marks)
13. (a) Draw the structure of compound N formed in the following reaction. (1 mark)
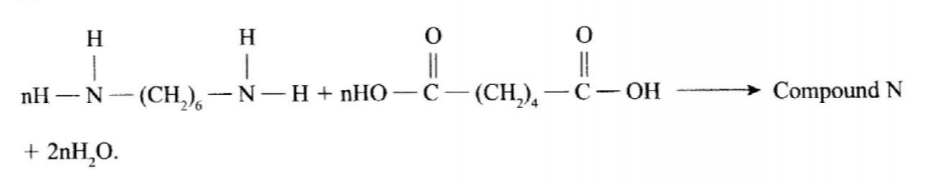
(b) Give one use of compound N. (1 mark)
14. When fuel bums in the internal combustion engine at high temperature, one of the products formed is nitrogen (11) oxide.
(a) Write the equation for the formation of nitrogen (II) oxide. (1 mark)
(b) Give a reason why nitrogen (II) oxide is not formed at room temperature. (1 mark)
(c) Describe how formation of nitrogen (H) oxide in the internal combustion engine leads to gaseous pollution. (2 marks)
15. Sodium hydroxide can be prepared by the following methods; I and II
1 Sodium metal -> Cold water -> sodium hydroxide + hydrogen
11 Concentrated -> Process A ->sodium hydroxide + chlorine + hydrogen Sodium chloride
(a) Name one precaution that needs to be taken in method I. (1 mark)
(b) Give the name of process A. (I mark)
(c) Give one use of sodium hydroxide. (I mark)
16. The atomic number of sulphur is 16.
Write the electron arrangement of sulphur in the following? (2 marks)
(a) H2(s)
(b) S032-
17. A compound whose general formula is M(OH)., reacts as shown by the equation.
M(OH)3 + OH–aq -> M(OH)3
M(OH)–4 (aq)+3H–(aq) ->+M3+(aq)
(a) What name is given to compounds which behave like M(01-1)3 in the two reactions. (1 mark)
(b) Name two elements whose hydroxides behave like that of M. (2 marks)
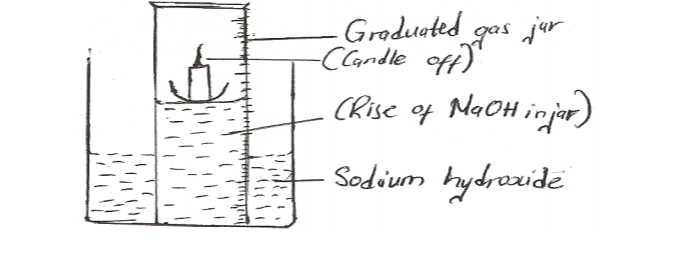
18. A water trough, aqueous sodium hydroxide, burning candle, watch glass and a graduated gas jar were used in an experimental set up to determine the percentage of active part of air.
Draw a labelled diagram of the set up at the end of the experiment. (3 marks)
19. In an experiment on rates of reaction, potassium carbonate was reacted with dilute sulphuric (VI) acid.
(a) What would be the effect of an increase in the concentration of the acid on the rate of the reaction? (1 mark)
(b) Explain why the rate of reaction is found to increase with temperature. (2 marks)
20. 60cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take 60cm3 sulphur (IV) oxide gas to diffuse through the same partition under the same condition? (S = 32.0, 0 = 16.0) (3 marks)
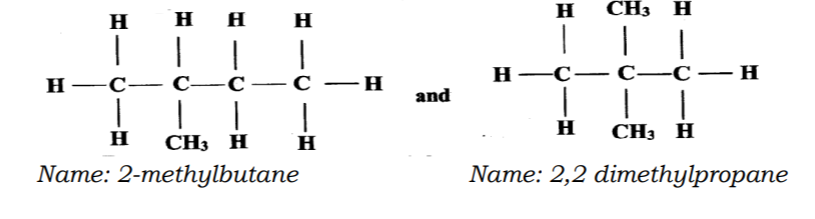
21. Draw and name the isomers of pentane. (3 marks)
22. The set up below was used to collect a dry sample of a gas.
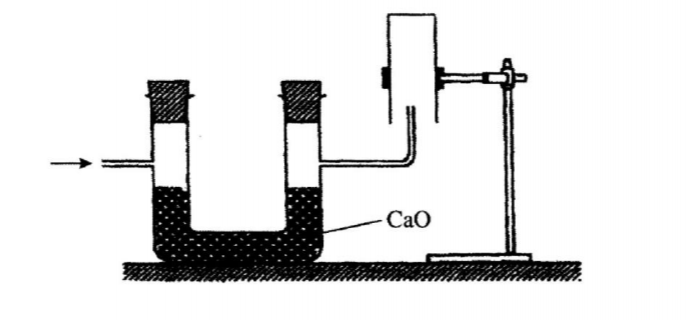
Give two reasons why the set up cannot be used to collect carbon (IV) oxide gas. (2 marks)
23. Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. (1 mark)
(b) Classify the substances in (a) above as acids bases or neutral. (2 marks)
24. Describe how a solid sample of potassium sulphate can be prepared starting with 200cm3 of 2M potassium hydroxide. (3 marks)
25. Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides. (a) Name the two oxides (2 marks)
(b) State one use of the two oxides. (1 mark)
26. Hydrogen sulphide is a highly toxic and flammable gas. It is normally prepared in a fume chamber.
(a) Name two reagents that can be used to prepare hydrogen sulphide in the laboratory. (1 mark)
(b) One of the uses of hydrogen sulphide is to produce sulphur as shown in the following equation.
2H2S(g) + SO2(g) + 2H2Og)) Identify the reducing agent in this reaction and give a reason for your answer. (1 mark) (c) Other than production of sulphuric (VI) acid.
State one commercial use of sulphur. (1 mark)
27. Describe an experimental procedure that can be used to extract oil from nut seeds. (2 marks)
28. A mixture contains ammonium chloride, Copper (11) oxide and sodium chloride.
Describe how each of the substances can be obtained from the mixture. (3 marks)
29. When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the student was relieved of pain. Explain. (2 marks)
| https://googleads.g.doubleclick.net/pagead/ads?client=ca-pub-0326674550430411&output=html&h=280&slotname=5740034820&adk=426665019&adf=1697964612&pi=t.ma~as.5740034820&w=336&lmt=1641281320&psa=1&format=336×280&url=https%3A%2F%2Fwww.advance-africa.com%2FKCSE-Past-Papers-2016-Chemistry-Paper-1.html&flash=0&wgl=1&dt=1641281319658&bpp=3&bdt=2902&idt=184&shv=r20211207&mjsv=m202112060101&ptt=9&saldr=aa&abxe=1&cookie=ID%3D87de00f0c876c980-22afddb0dcce0092%3AT%3D1640760436%3ART%3D1640760436%3AS%3DALNI_MbO5KvUEkn_9Lf85NifGlSAHi4D8A&prev_fmts=0x0%2C336x280%2C336x280&nras=1&correlator=2615810678553&frm=20&pv=1&ga_vid=771581788.1594825585&ga_sid=1641281320&ga_hid=885010326&ga_fc=1&u_tz=180&u_his=1&u_h=900&u_w=1600&u_ah=860&u_aw=1600&u_cd=24&u_sd=1&adx=565&ady=8304&biw=1583&bih=747&scr_x=0&scr_y=0&eid=44750774%2C182982000%2C182982200%2C31063824&oid=2&pvsid=532407994112837&pem=734&tmod=594&ref=https%3A%2F%2Fwww.advance-africa.com%2FKCSE-Past-Papers.html&eae=0&fc=1920&brdim=-8%2C-8%2C-8%2C-8%2C1600%2C0%2C1616%2C876%2C1600%2C747&vis=1&rsz=d%7C%7CEebr%7C&abl=CS&pfx=0&fu=0&bc=31&ifi=4&uci=a!4&btvi=1&fsb=1&xpc=RFpLC0p1wR&p=https%3A//www.advance-africa.com&dtd=492 |
Questions and Answers
2016 Chemistry paper 1
No. 1. A student investigated the effect of an electric current by passing it through some substances. The student used inert electrodes, and connected a bulb to the circuit. The table below shows the substances used and their states.
Experiment Substance State

(a) In which experiment did the bulb not light? (1 mark)
❖ 1 and 3
(b) Explain your answer in (a) above. (2 marks)
❖ In 1 ions K2CO3 are held rigidly within the crystal cannot move (no mobile ions) In 3 sugar exist as molecule hence no mobile ions.
No.2.An alknal has the following composition by mass: hydrogen 13.5%, oxygen 21.6% and carbon 64.9%
(a)Determine the empirical formula of the alcohol(C=12.0; H=1.0′ 0=16.0). (2mks)
❖ [E.F.= C4H9
(b)Given that empirical formula and the molecular formula of the alkanol are the same,draw the structure of the alkonol
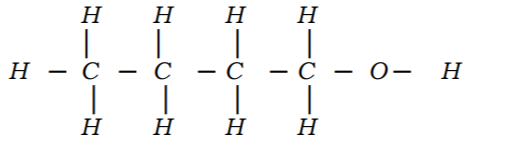
No. 3. The figure below shows an energy cycle.
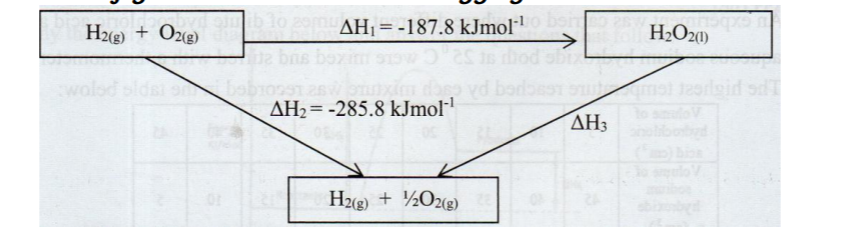
Give the name of the enthalpy change AH1. (1 mark)
❖ Enthalpy of formation of hydrogen peroxide or enthalpy of formation
(b)Determine the value of AH3.(2 marks)
❖ AFT1 + AH3 = AH2‘ > AH2 = AH2 – AFli ❖ = – 285.8 – (-187.8) = 187.8 – 285.8 = – 98 kJmo1-1
4.The set up below was used to investigate the reaction between dry hydrogen gas and copper
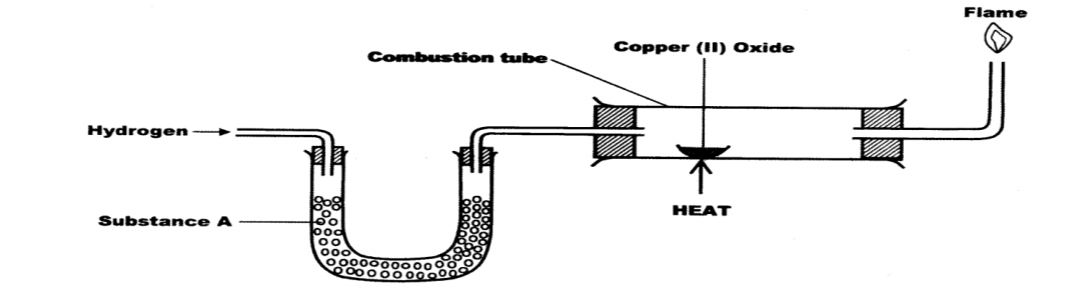
(a) Name substance A.(1 mark)
❖ Fused anhydrous calcium chloride
❖ Cao: fused CaCl2
(b) State the observation made in the combustion tube. (1 mark)
❖ Black CuO changes to brown Cu metal
❖ Formation of colourless liquid on the cooler parts of the combustion tube.
(c) Explain the observation stated in (b) above. (1 mark)
❖ Copper (II) oxide is reduced by hydrogen to copper metal while hydrogen is oxidized to water / CuO reduced to Cu /H2 Oxidized to H2O
No. 5. Starting with sodium metal, describe how a sample of crystals of sodium hydrogen carbonate may be prepared. (3 marks)
❖ React sodium with water to get sodium hydroxide. VBubble into this solul excess carbon (IV) oxide to get sodium hydrogen carbonate
No. 6. Ammonium ion has the following structure

4. Label on the structure: (a) covalent bond; (b) coordinate (dative) bond. (a) and (b)Dath bond
Covalest bond
No. 7.When 8.53g of sodium nitrate were heated mass of oxygen gas produced was 0.83 g.
Given reaction as 2NaNO3 (s) -> 2NaNO2 (s) + 0 2 (g) Calculate the percentage of sodium nitrate that nitrite. (Na = 23.0, N = 14.0, 0 = 16.0) (3 marks)
in an open test-tube, the the equation of the was converted to sodium

No.8. Aluminium is both malleable and ductile.
(a)What is meant by?
(i) Malleable: (1 mark)
❖Can be hammered into sheets.
(ii)Ductile (1 mark)
❖ Can be drawn into wires.
(b)State One use of aluminium based on: (i)malleability (1/2 marks) ❖ Making of sufurias/ motor vehicle parts/ aeroplane parts, window / door flames, cups, plates, packaging materials, pans, making sheets/ roof
(ii)ductility (1/2 marks)
❖ electricity cables/ wires.
No. 9.The diagram below represents the set-up that was used to prepare and collect hydrogen chloride gas in the laboratory.
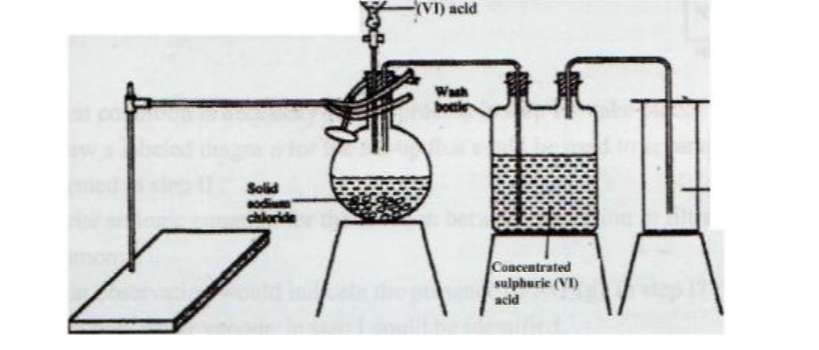
State the purpose of concentrated sulphuric (VI) acid in the wash bottle. (1 mark)
❖ It is a drying agent.
Write an equation for the reaction between dry hydrogen chloride gas and heated iron. (1 mark)
❖ Few + 2HCI(g) FeCl2(S) +H2(g)
No. 10 . Iron (III) oxide was found to be contaminated with copper (H) sulphate. Describe how a pure sample of iron (HI) oxide can be obtained. (3 marks)
❖ Add water to dissolve CUSO4, Fe2O3 doesn’t dissolve
❖ Filter out the undissolved Fe2O3
❖ Wash the residue with plenty of water to remove traces of the filtrate.
❖ Dry the residue between the filter papers
No.11.a) Complete the nuclear equation below: (1 mark)
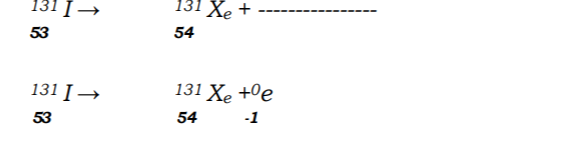
(b)The half-life of I is 8 days. Determine the mass of 1311 remaining if 50 grammes decayed for 40 days (1 mark)

(c)Give one harmful effect of radioisotopes.
❖ Instant / cause death
❖ Cause cancer
❖ Cause gene mutation (1 mark)
No. 12. During an experiment, chlorine gas was bubbled into a solution of potassium iodide. (a)State the observations made. (1 mark)
❖ Solution turned from colourless to dark brown
❖ Greenish yellow / pale green colour of C12 disappears
❖ Brown solution / black solid is deposited
(b)Using an ionic equation, explain why the reaction is redoes. (2 marks)
❖ C12 (aq) +21 – (aq) — 12 (aq).2CI- (aq)
❖ Explanation; Iodine oxidation state changes from -1 to 0 hence oxidation while Cl2 0.5 changes from 0 to -1 hence reduction / increase is ON and decrease is ON or movement of electrons Cl2 gains e’s where lose
No.13.(a)Draw the structure of compound N formed in the following reaction.
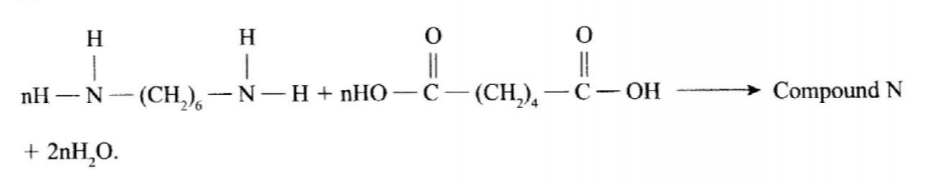
(b) Give one use of compound N. (l Mark)
❖ Making synthetic fibres such as for: – Ropes
❖ Blouses
❖ Stockings
❖ Undergarments Trousers
No. 14.When fuels burn in the internal combustion engine at high temperature, one of the products formed is nitrogen (II) oxide.
(a) Write the equation for the formation of nitrogen (II) oxide. (1 mark)
❖ N2g) + 02(g) -) 2N0(g)
(b) Give a reason why nitrogen (II) oxide is not formed at room temperature. (1 mark)
❖ Nitrogen atoms in the molecule are joined by strong triple covalent bond that requires a lot of energy to break than provided at room temperature
(c) Describe how formation of nitrogen (H) oxide in the internal combustion engine leads to gaseous pollution. (2 mark)
❖ Nitrogen (II) oxide reacts with oxygen in air to form nitrogen (IV) oxide that dissolves in water vapour causing acid rain.
15.Sodium hydroxide can be prepared by the following methods; I and II. I. Sodium metal cold water Sodium hydroxide + Hydrogen II. Concentrated Process A Sodium hydroxide + Chlorine + Hydrogen Sodium chloride
(a) Name one precaution that needs to be taken in method I.
❖ Small piece of sodium metal (pea size) with a lot of water
❖ Perform the experiment wearing goggles.
(b) Give the name of process A. (1 mark)
❖ Electrolysis (1 mark)
(c) Give one use of sodium hydroxide. (1 mark)
❖ Manufacture of paper (soften), soaps and detergents
❖ Fractional distillation of liquid air
❖ Extraction of aluminium metal
❖ Manufacture of bleaching agents eg Na0Cl paper, textiles, oil refinery
❖ Making herbicides on weed killers
❖ Textile industry to soften
No. 16.The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the following: (2 marks)
(a) H2S
❖2.8.8
(b) SO2-3
❖ 2.8.2
No. 17. A compound whose general formula is M(OH)3 reacts as shown by the equation below.
M (OH) 3(s) + OH4 (aq)
M(OH)3(aq)
M (OH) 3(s) + 3H+ (aq) —10″ M (aq) + 3H20 (i)
(a) What name is given to compounds which behave like M(OH)3 in the two reactions. (1 mark)
❖ Amphoteric
(b) Name two elements whose hydroxides behave like that of M. (2 marks)
❖ Lead, Zinc and Aluminium
No.18.A water trough, aqueous sodium hydroxide, burning candle, watch class and a graduated gas jar were used in an experimental set up to determine the percentage of active part of air. Draw a labeled diagram of the set up at the end of the experiment.
No. 19.In an experiment on rates of reaction, potassium carbonate was reacted with dilute sulphuric (VI) acid.
(a) What would be the effect of an increase in the concentration of the acid on the rate of the reaction? (1 mark) ❖ The rate of reaction increases. This is because when the concentration is high: the number of collisions between particles is also high hence reacts faster,
No. 20. 60 cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take 60cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the same conditions? (S = 32.0, 0 = 16.0) (3 marks)

No. 21. Draw and name the isomers of pentane.(3 marks)
No. 22.The set-up below was used to collect a dry sample of a gas
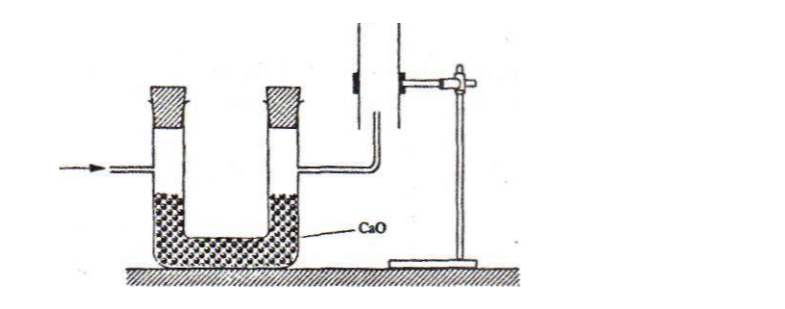
Give two reasons why the set-up cannot be used to collect carbon (IV) oxide gas. (2 marks)
❖ It is denser than air
❖ It will react with calcium oxide since CO2 is acidic and CaO is basic
No. 23. Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. (1 mark)
(b) Classify the substances in 15(a) above as acids, bases or neutral. (2 marks)
No. 24. Describe how a solid sample of potassium sulphate can be prepared starting with 200cm3of 2M potassium hydroxide. (3 marks)
❖ Vol of 2KOH = 100cm3 (or mols = 0.4 = 0.2
❖ Mix the KOH(aq) and H2SO4 acid
❖ Concentrate the mixture/ heat the mixture
❖ Crystalise the solution ( or heating the solution to dryness)
❖ Dry crystals
No. 25. Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides. (a) Name the two oxides. (2 marks)
❖ Carbon (IV) oxide /CO2/ carbon dioxide
❖ Carbon (II) oxide/ CO/ carbon monoxide
(b) State one use of the two oxides. (1 mark)
• Fire extinguisher/ photosynthesis
❖Refrigeration
❖ Solvay process
❖ Fizzy drinks
❖ Food preservation
❖ Extraction of metals
❖ Manufacture of methanol
❖ Manufacture of fuel (water, gas)
NO. 26.Hydrogen sulphide is a highly toxic and flammable gas. It is normally prepared in a fume chamber. (a) Name two reagents that can be used to prepare hydrogen sulphide in the laboratory.(1 mark)
❖ Iron (II) sulphide or conc sulphide / copper sulphide (Accp formula: Fes/ HCl)
❖ Hydrochloric acid or lead (II) sulphide/ HNO3
(b) One of the uses of hydrogen sulphide is to produce sulphur as shown in the following equation:
2H2S(g) + SO2 (g) -> 3S(s) + 2H20(1) Identify the reducing agent in this reaction and give a reason for your answer. (1 mark)
❖ Hydrogen sulphide
❖ The sulphur changes from -2 to zero/ (it reduces SO2 to S) i.e. +4 to 0 / sulphur lost e’s in the H2S to form. sulphur
(c) Other than production of sulphuric (VI) acid, state one commercial use of sulphur.(1 mark)
❖ Vulcanization of rubber
❖ Manufacture of sulphur drugs
❖ Manufacture
of gun powder/ match sticks / explosives/ fungicides No. 27. Describe an experimental procedure that can be used to extract oil from nut seeds. (2 marks)
❖ Crush grind using a pestle and mortar, add suitable solvent of propanone ethanol alcohol and stir to dissolve oil. Filter the mixture to obtain a solution of the oil. Leave the solution in the sun for propanode to evaporate leaving the oil.
No. 28 . A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained form the mixture. (3 marks)
❖ Heat the mixture to sublime the NH4Cl. Add water to dissolve the NaCl. Copper (II) oxide does not dissolve. Filter and evaporate the filtrate to obtain sodium chloride.
No. 29 . When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the student was relieved of pain. Explain. (2 marks)
❖ The product from nettle plant is acidic; aqueous ammonia solution being basic neutralize the acidic product

