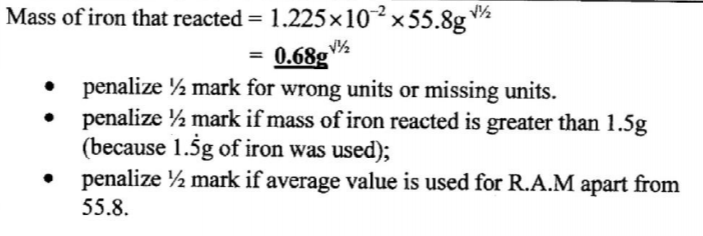2017 KCSE Chemistry Paper 3 Past Paper
1. You are provided with:
• Solution A, 0.5 M copper(II) sulphate
• Solid B1, metal B1 powder
• Solid B2, Iron powder
• Solution C, 0.02 M acidified potassium manganate(VII)
You are required to determine the:
• Enthalpy change for the displacement reaction between metal B1 and copper(II) sulphate.
• Mass of iron that reacts with copper(II) sulphate in the displacement reaction.
PROCEDURE I
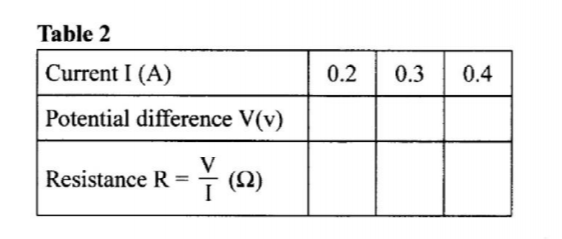
(a) (i) Using a pipette and a pipette filler, place 25.0 cm3 of solution A into a 100 m plastic beaker.
Allow to stand for about 1 minute and then measure the temperature of the solution.
Record the reading in Table 1 as the initial temperature.
Add al of solid B1 to the solution.
Stir the mixture carefully with the thermometer an measure the highest temperature reached.
This will take about 5 minutes.
Recoil the reading in Table 1 as maximum temperature reached.
(ii) Calculate the:
I number of moles of copper(II) sulphate used. (1 mark)
II enthalpy change for the reaction of metal B1 with one mole of copper(II) sulphate. (Assume that for the mixture, specific heat capacity = 4.2 Jg-‘K-1 and density = 1.0 g cm-3) (1 mark)
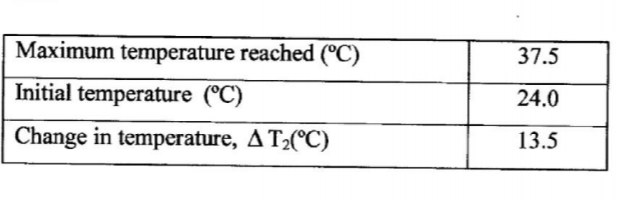
(b) Repeat procedure I, (a) (i) with all of metal B2 (iron powder) in place of metal B1. The maximum temperature is reached after about 8 minutes.
Record the temperature readings in Table 2. Retain the mixture for use in PROCEDURE II.
(c) Compare the changes in temperature T1 and T2 and comment on the differences. (2 marks)
PROCEDURE II
(i) Fill a burette with solution C.
(ii) Filter the mixture obtained in procedure I (b) into a 250 ml volumetric flask. Wash the residue with distilled water and add into the flask. Add more distilled water to make up to the mark. Label this as solution B2.
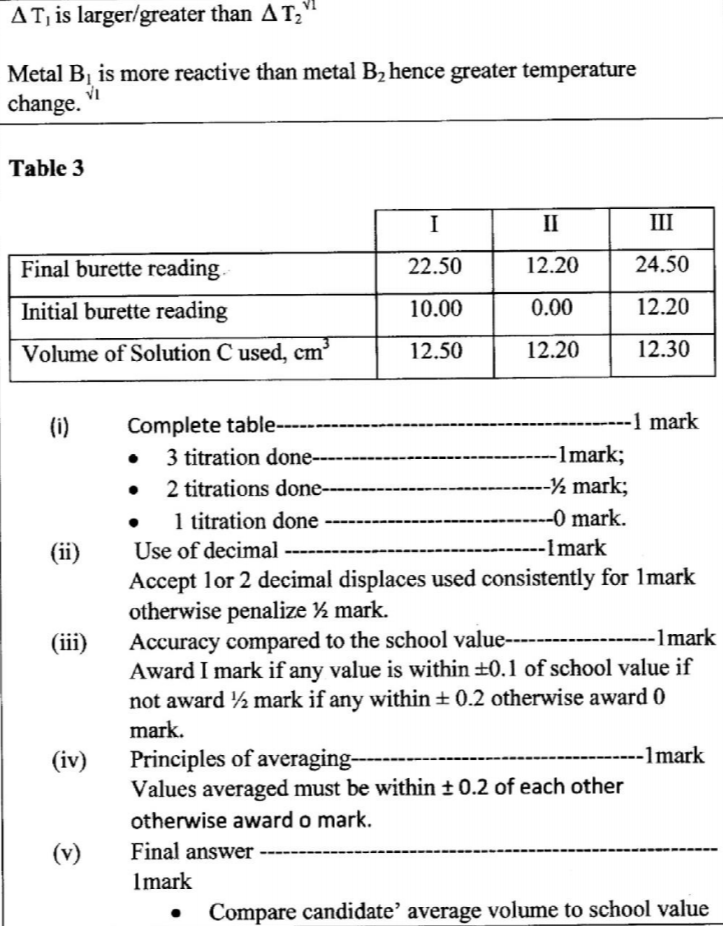
(iii) Using a pipette and a pipette filler, place 25.0 cm3 of solution B2 into a 250 ml conical flask. Titrate solution B2 with solution C until a permanent pink colour just appears. Record the readings in Table 3.
Repeat step (iii) and complete Table 3.
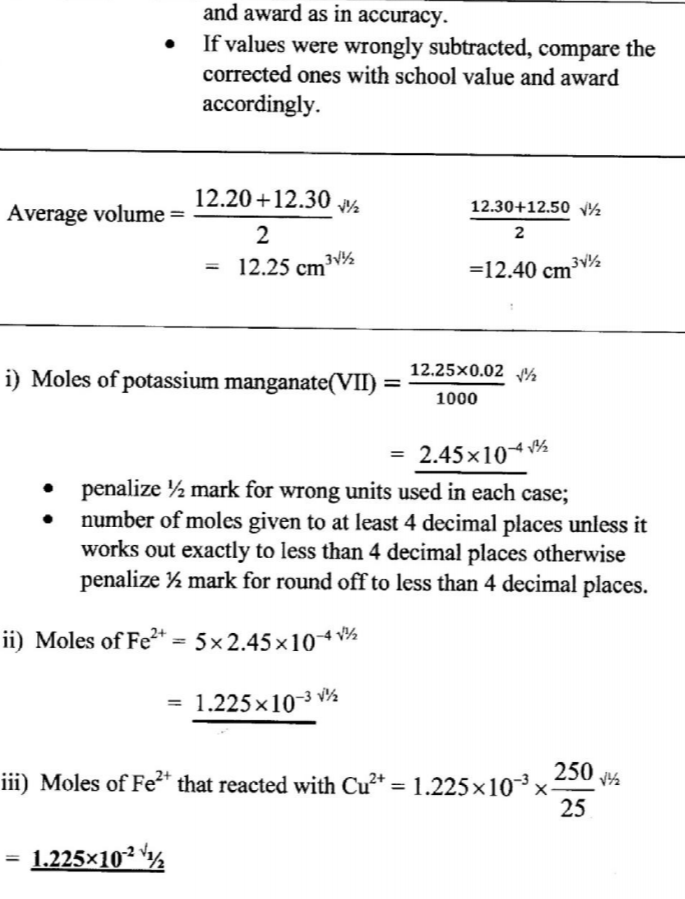
(e) Calculate the average volume of solution C used (1 mark)
(f) The equation for the reaction between manganate(VII) and iron(II) ions is:
Mn04(aq) + 5Fe2+(aq) + 8H+(aq) > Mn2+(aq) + 5Fe3+(aq) + 4H20(aq)
Calculate the number of moles of: (i) potassium manganate(VII) used. (1 mark)
(ii) iron (II) ions in 25.0 cm3 solution B (1 mark)
(iii) iron that reacted with copper(I1) sulphate. (1 mark)
(g) Determine the mass of iron that reacted. (RAM of Fe = 55.8) (1 mark)
2. You are provided with:
• Solid K
• Aqueous ammonia
• Aqueous sodium sulphate
• Dilute nitric(V) acid
• Wooden splint
Solid K is suspected to be lead(II) carbonate.
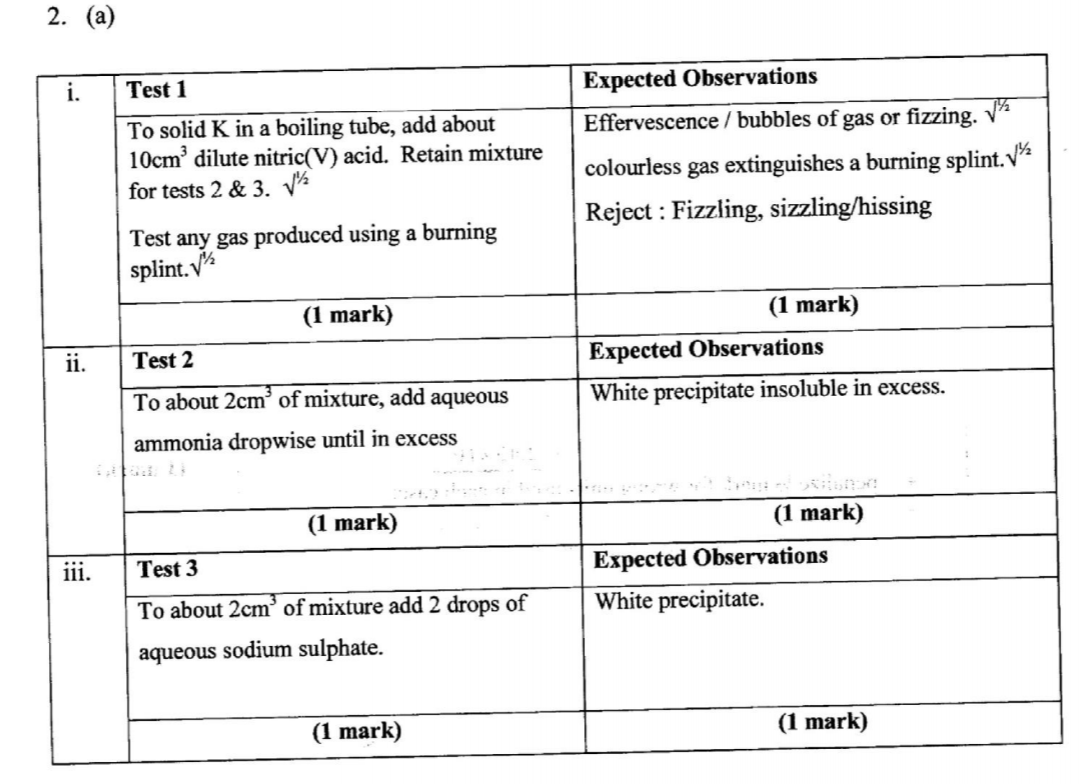
(a) From the reagents provided, select and describe three tests that could be carried out consecutively to confirm if solid K is lead(II) carbonate. Write the tests and expected observations in the places provided.
(b) Carry out the tests described in (a) above
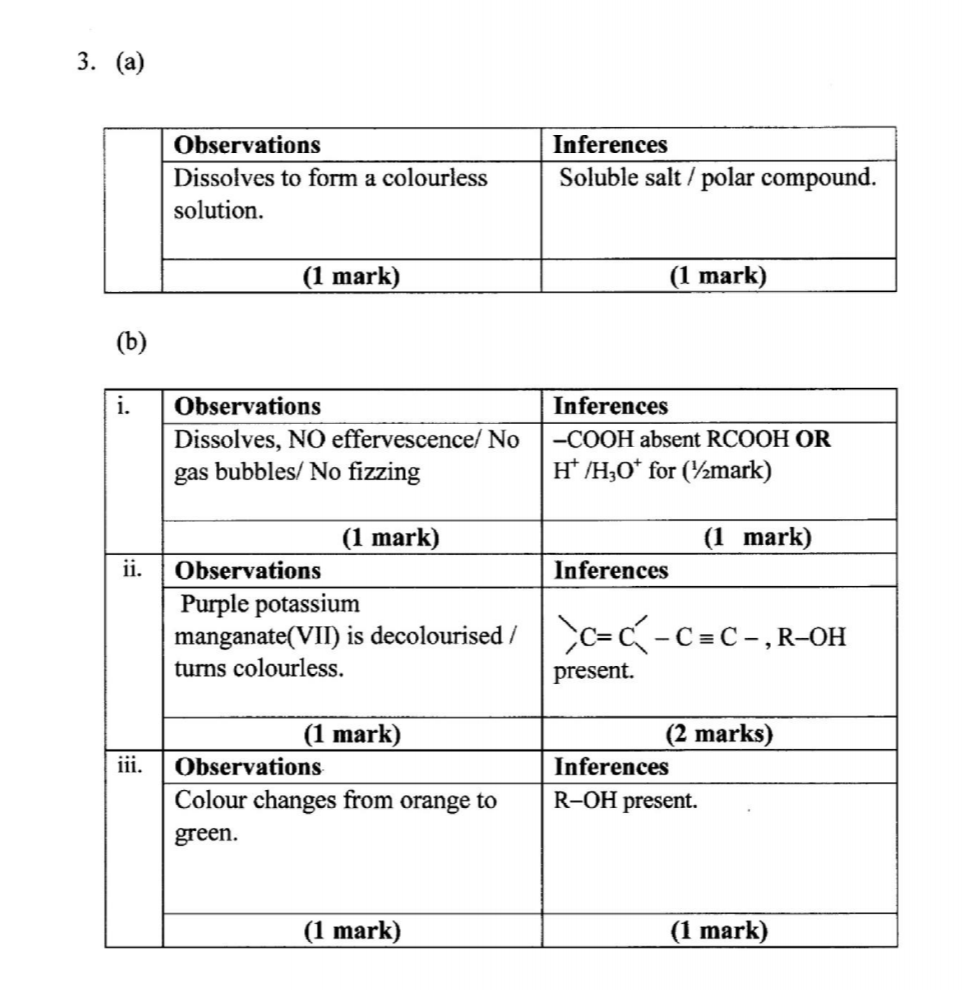
3. You are provided with an organic compound solid M. Carry out the following tests. Record the observations and inferences in the spaces provided.
(a) Place all of solid M in a boiling tube. Add about 10 cm3 of distilled water and shake. Retain the solution for use in procedure (b) (i), (ii) and (iii).
Use about 2 cm3 portions of the mixture in a test tube for tests (i), (ii) and (iii).
(i) To the first portion, add all the solid sodium carbonate provided.

(ii) To the second portion, add two drops of acidified potassium manganate(VII) and warm the mixture.

(iii) To the third portion, add about 2 cm3 of acidified potassium dichromate(V1). Heat the mixture to boiling and allow to stand for about 2 minutes.

2017 KCSE Chemistry Paper 3 Past Paper-Marking Scheme/Answers
1.
b).
c).
d).