2019 KCSE Chemistry paper 2 Past Paper
1. (a) Alkanes are said to be saturated hydrocarbons.
(i) What is meant by saturatcd hydrocarbons. (1 mark)
(ii) Draw the strucuire of the third member of the alkane homologous series and name it. (2 marks)
(b) When the alkane, hexane, is heated to high temperature, one of the products is ethene.
(i) Write the equation for the reaction. (1 marks)
(ii) Name the process described in (b). (1 marks)
(c) Study the flow chart in Figure 1 and answer the questions that follow.
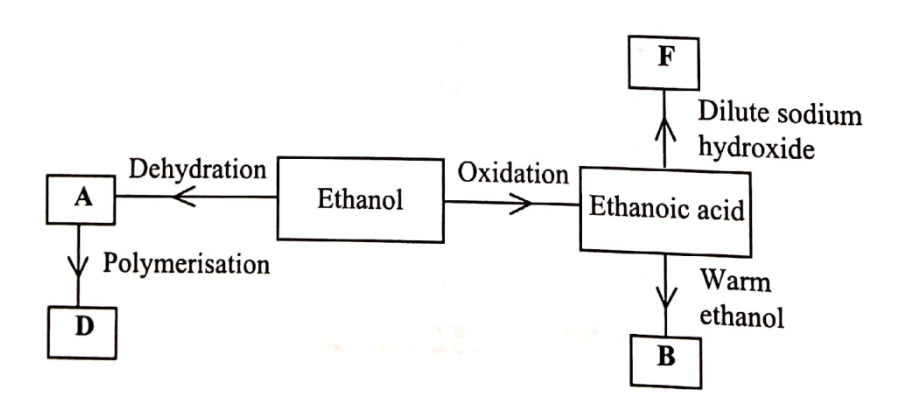
(i) Identify A. (1 marks)
(ii) State one physical property of B. (1 marks)
(iii) Draw the structure of D. (1 marks)
(iv) Give a reason why D pollutes the environment.(1 marks)
(v) Write an equation for the formation of F.(1 marks)
(d) Describe an experiment which can be used to distinguish butene from butanol. (2 marks)
2. (a) Zinc occurs mainly as zinc blende. Name one other ore from Which zinc can be extracted.(1 marks)
(b) The flow chart in Figure 2 shows the various stages in the extractİoil of zinc metal. Study it and answer the questions that follow.
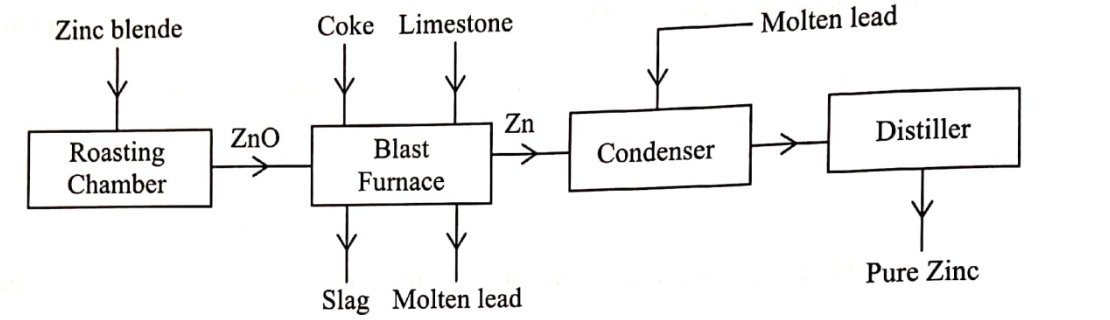
(i) Write an equation for the reaction which occurs in the roasting chamber. (1 mark)
(ii) Describe the process that takes place in the blast furnace. (3 mark)
(iii) Explain why molten lead is added to the condenser. (1 mark)
(iV) State two uses of zinc.(1 mark)
(v) Give one reason why the extraction of zinc causes pollution to the environment.(1 mark)
(c) Explain the observations made when zinc metal is added to hot sodium hydroxide. (2 marks)
3. Figure 3 is a flow chart that shows the proccss that occurs in the manufacture of nitric(v)acid
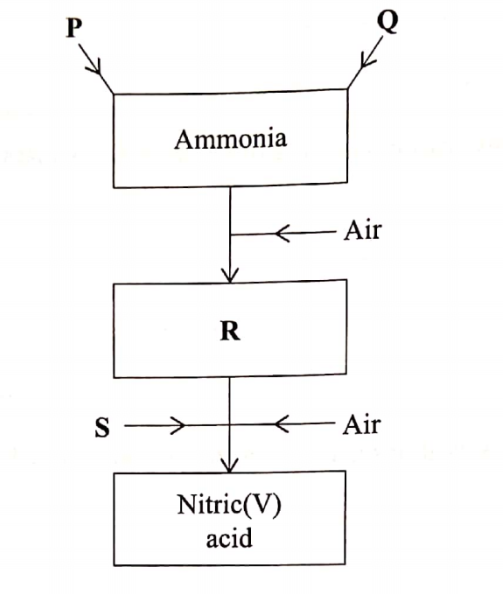
(a) Name substance P, Q, R and S. P……………………… (1 marks)
Q………………………… (1 marks)
R…………………………..(1 marks)
S…………………………….(1 marks)
(b) To obtain substance R, ammonia is heated at 900 °C in the presence of air and a catalyst.The product is then cooled in air.
(i) Name the catalyst for the reaction. (1 marks)
(ii) Write the equations for the two reactions described in (b). (2 marks)
(iii) Other than nitric(V) acid, name another product that is formed. (1 mark)
(c) When ammonia is reacted with nitric(V) acid, it produces a nitrogenous fertiliser.
(i) Explain why fertilisers play a major role in food production. (2 marks)
(ii) State two problems associated with the use of nitrogenous fertilisers. (2 marks)
4. (a) Explain the following observations:
(i) The colour of aqueous copper(ii) sulphate fades when a piece of magnesium metal is droppcd into the solution. (2 marks)
(ii) A piece of iron bar is coated with a brown substance when left in the open on a rainy day. (2 marks)
(b) A sample of water is suspected to contain aluminium ions (AI3+) .
Describe a laboratory experiment that can be carried out to show that AI3+ ions are present in the water sample. (3 marks)
(c) In an experiment to determine the number of moles of water of crystallisation of a hydrated compound Na2SO4.XHsO,5g of the compound were heated strongly to a constant mass.
(i) Explain how a constant mass was obtained. (2 marks)
(ii) During the experiment, the mass of the residue was found to be 2.205 g.
Determine the number of moles of water of crystallisation in the compound. (Na = 23.0 ; O = 16.0 ; S = 32.0 ; H = 1.0) (3 marks)
5. (a) What is meant by a molar heat of neutralisation? (1 marks)
(b) In an experiment to determine the molar heat of neutralisation, 50 cm3 of IM hydrochloric acid was neutralised by adding 10 cm3 Onions Of dilute sodium hydroxide.
During the experiment, the data in Table 1 was obtained.
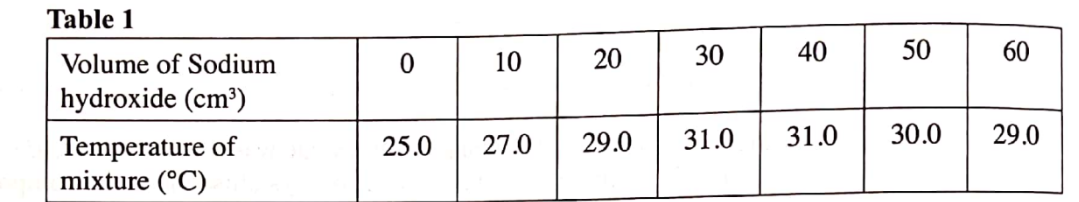
(i) Write the equation for the reaction in this experiment. (1 mark)
(ii) On the grid provided, plot a graph of temperature (Y-axis) against volume of sodium hydroxide (X-axis) added. (3 marks)
(iii) Determine from the graph the:
I. volume of sodium hydroxide which completely neutralises 50 cm3 of lM hydrochloric acid. (1 mark)
11. change in temperature, AT, when complete neutralisation occurred. (1 mark)
(IV) Calculate:
I. The heat change, dH when complete neutralisation occurred. (Specific heat capacity = 4.2 Jg-1 K-1 density of solution 1.0 gcm-3)(2 marks)
(v) How would the value of molar heat differ if 50 cm3 of lM ethanoic acid was used instead of IM hydrochloric acid? Give a reason. (2 marks)
6. (a) What is meant by standard electrode potential of an element (1 mark)
(b) Use the standard electrode potcntials given below to answer the questions that follow.
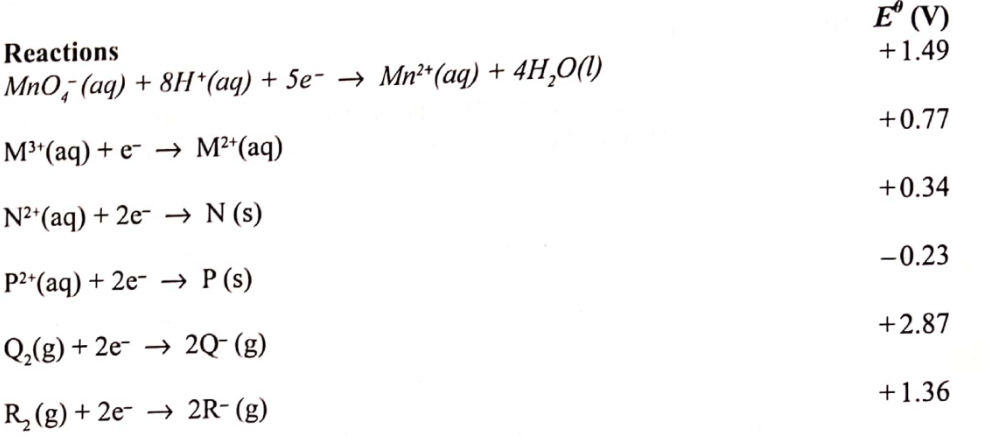
(i) State whether acidified MnO–4 can oxidise M2+. Give a reason. (2 marks)
(ii) Select two half-cells which when combined will give the highest e.m.f. (1 mark)
(iii) Write the cell representation for the cell formed in b (ii). (1 mark)
(iv) Calculate the Eθ value for the cell formed in b (iii). (1 mark)
(c) A mass of 1.24g of a divalent metal was deposited when a current of 6A was passed through a solution of a metal sulphate for 12 minutes.Determine the relative atomic mass of the metal( Faraday = 96,500 C mol -1 (3 mark)
(d) State two application s of electrolysis. (I mark)
7. (a) What is meant by rate of reaction (1 mark)
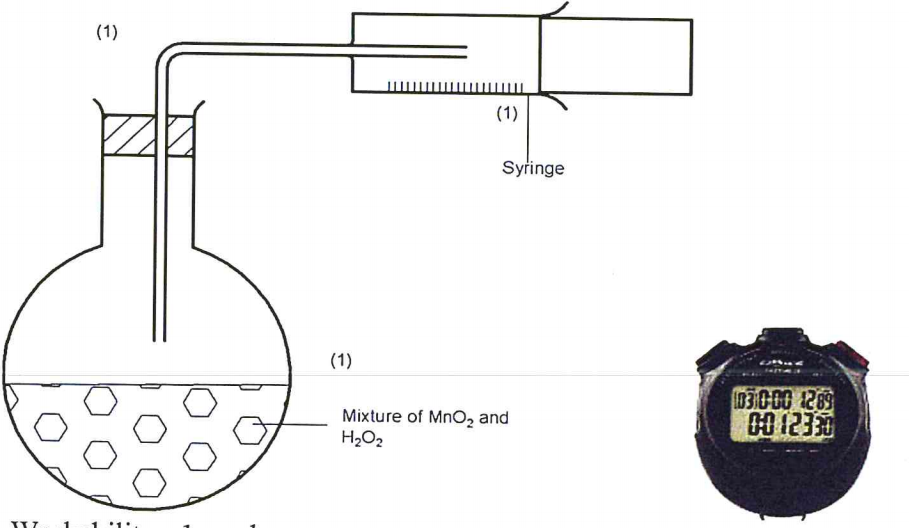
(b) In the space provided, Sketch the diagram of a set-up that Can be used to determine the rate of reaction between manganese(IV) oxide and hydrogen peroxide. (3 marks)
(c) A student placed a small amount of liquid bromine at the bottom of a sealed gas jar of air as shown in Figure 4.
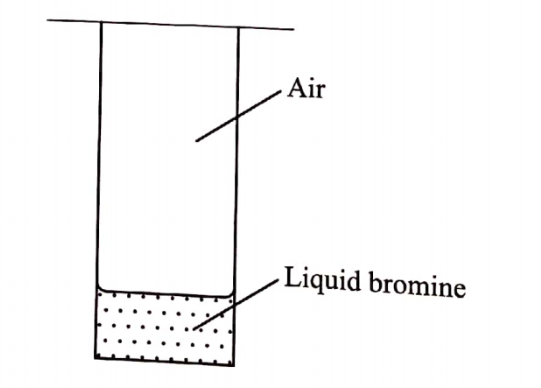
(i) Describe what will be observed: (1 mark)
I. After two minutes………………………….
II. After 30 minutes ……………………..
(ii) Use the Kinetic theory to explain the observations: (2 marks)
I. After two minutes………………………….
II. After 30 minutes ……………………..
(d) Some plants have seeds that contain vegetable oil.
(i) Describe how the oil can be obtained from the seeds. (3 marks)
(ii) Explain how it could be confirmed that the liquid obtained from the seeds is oil. (1 mark)
2019 KCSE Chemistry paper 2 Past Paper-Marking Scheme/Answers
1. (a) Alkanes are said to be saturated hydrocarbons.
(i) What is meant by saturated hydrocarbons. (1 mark)
Compounds made of carbon and hydrogen only and where the bonds (carbon to carbon) are single covalent /every carbon atom contains its maximum number of hydrogen atoms.
(ii) Draw the structure of the third member of the alkane homologous series and name it. (2 marks)
Propane
(b) When the alkane, hexane, is heated to high temperature, one of the products is ethene.
(i) Write the equation for the reaction. (1 marks)C6HI4C2H4+C4H10
(ii) Name the process described in (b). (1 marks)Thermal Caracking
(c) Study the flow chart in Figure 1 and answer the questions that follow.

(i) Identify A. (1 marks)
ethene / CH2CH2
(ii) State one physical property of B. (1 marks)Sweet/pleasant smell / fruity smell Miscible /solub1e in water
(iii) Draw the structure of D. (1 marks)
(iv) Give a reason why D pollutes the environment.(1 marks)It is non-biodegradable
(v) Write an equation for the formation of F.(1 marks)
(d) Describe an experiment which can be used to distinguish butene from butanol. (2 marks) Add bromine water to both yellow colour of bromine persists in butanol butene decolourises bromine water.
2. (a) Zinc occurs mainly as zinc blende. Name one other ore from Which zinc can be extracted.(1 marks) ZnCO3/Zinc Carbonate, ZnO/Zinc Oxide
(b) The flow chart in Figure 2 shows the various stages in the extraction of zinc metal. Study it and answer the questions that follow.

(i) Write an equation for the reaction which occurs in the roasting chamber. (1 mark)
2ZnS+ 30 (g) —› 2ZnO(s)+2SO,(g)
(ii) Describe the process that takes place in the blast furnace. (3 mark)
– Decomposition of calcium carbonate/ formation of oxides of carbon; – Reduction of zinc oxide; – Formation of calcium silicate; – Formation of slag
(iii) Explain why molten lead is added to the condenser. (1 mark)
Lead is more reactive than zinc hence, the coating protects it from re-oxidation/ it acts as a coolant.
(iv) State two uses of zinc.(1 mark)
(v) Give one reason why the extraction of zinc causes pollution to the environment.(1 mark)Gullies are formed; Emission of by-product sulphur(IV) oxide and carbon(II) oxide which are poisonous; Causes acid rain formation; Carbon(IV) oxide causes global warming; lead is poisonous.
(c) Explain the observations made when zinc metal is added to hot sodium hydroxide. (2 marks)Zinc reacts with hot sodium hydroxide to form Zincate because Zinc is amphoteric ORZinc dissolves in sodium hydroxide to form a solution Bubbles of a colourless gas formed as zinc reacts with steam
3. Figure 3 is a flow chart that shows the proccess that occurs in the manufacture of nitric(v)acid

(a) Name substance P, Q, R and S.P – Nitrogen Q- Hydrogen R – Nitrogen(11) oxide S – Water
(b) To obtain substance R, ammonia is heated at 900 °C in the presence of air and a catalyst.The product is then cooled in air.
(i) Name the catalyst for the reaction. (1 marks)
Platinum/Rhodium (Pt/Rh)
(ii) Write the equations for the two reactions described in (b). (2 marks)
4NH,(g)+ 3O2(g) —+ 4NO(g) +6H2O(1)
(iii) Other than nitric(V) acid, name another product that is formed. (1 mark)
(c) When ammonia is reacted with nitric(V) acid, it produces a nitrogenous fertiliser.
2NO(g) + 02 (g) —+ 2NO
(i) Explain why fertilisers play a major role in food production. (2 marks)Fertilizers improve the soil fertility; Adds nutrients to the soil; increases food productivity
(ii) State two problems associated with the use of nitrogenous fertilisers. (2 marks)
• Excess nitrates in drinking water causes stomach upsets
• When nitrates are converted to nitrites by bacteria, the nitrites react with blood affecting its ability to react with oxygen
• Eutrophication
4. (a) Explain the following observations:
(i) The colour of aqueous copper(ii) sulphate fades when a piece of magnesium metal is dropped into the solution. (2 marks)
The blue colour is due to presence of (Cu 2+) ions.Magnesium is more reactive than copper, therefore it displaces copper (Cu 2+) ions from solution.
(ii) A piece of iron bar is coated with a brown substance when left in the open on a rainy day. (2 marks)
In the presence of air (oxygen) and water , iron is oxidized to hydrated iron (III) oxide which is the brown porous substance.
(b) A sample of water is suspected to contain aluminium ions (AI3+) .
Describe a laboratory experiment that can be carried out to show that AI3+ ions are present in the water sample. (3 marks)
Add excess aqueous ammonia to the water, formation of white precipitate shows presence of Al” or Pb2+ ions
(c) In an experiment to determine the number of moles of water of crystallisation of a hydrated compound Na2SO4.XHsO,5g of the compound were heated strongly to a constant mass.
(i) Explain how a constant mass was obtained. (2 marks)
To another sample of water add dilute H2SO4 / HC1 formation of colouress solution shows presence of Al°° / add potassium iodide if a yellow precipitate does not form it confirms presence of Al’”ions. (ii) During the experiment, the mass of the residue was found to be 2.205 g.
Determine the number of moles of water of crystallisation in the compound. (Na = 23.0 ; O = 16.0 ; S = 32.0 ; H = 1.0) (3 marks)
5. (a) What is meant by a molar heat of neutralisation? (1 marks)
Heat change that occurs when one mole of OH‘ ions reacts with one mole of H+ ions to form 1 mole of water.
(b) In an experiment to determine the molar heat of neutralisation, 50 cm3 of IM hydrochloric acid was neutralised by adding 10 cm3 Onions Of dilute sodium hydroxide.
During the experiment, the data in Table 1 was obtained.

(i) Write the equation for the reaction in this experiment. (1 mark)
NaOH(aq)+ HC1(aq) = NaC1(aq) + H2O(1)
(ii) On the grid provided, plot a graph of temperature (Y-axis) against volume of sodium hydroxide (X-axis) added. (3 marks)

(iii) Determine from the graph the:
I. volume of sodium hydroxide which completely neutralises 50 cm3 of lM hydrochloric acid. (1 mark)
35.5cm3
ii. change in temperature, AT, when complete neutralisation occurred. (1 mark)
-25
(IV) Calculate:
I. The heat change, dH when complete neutralisation occurred. (Specific heat capacity = 4.2 Jg-1 K-1 density of solution 1.0 gcm-3)(2 marks)
= 2332.155 J
II. Molar heat of neutralisation of hydrochloric acid with sodium hydroxide. (1 mark)
Moles of HCL 50X1/1000
0.05 moles = 2332.2
1mole = 2332.2/0.05
= – 46.64kJmo1
(v) How would the value of molar heat differ if 50 cm3 of lM ethanoic acid was used instead of IM hydrochloric acid? Give a reason. (2 marks)
The molar heat of ethanoic acid would be lower. Ethanoic acid is a weak acid and thus some of the heat energy is utilized in ionizing the acid.
6. (a) What is meant by standard electrode potential of an element (1 mark)
This is the potential difference between a standard hydrogen half- cell and a half-cell of an element containing 1mo1dm-3 of the ions at 298 K and 1 atmospheric pressure
(b) Use the standard electrode potentials given below to answer the questions that follow.
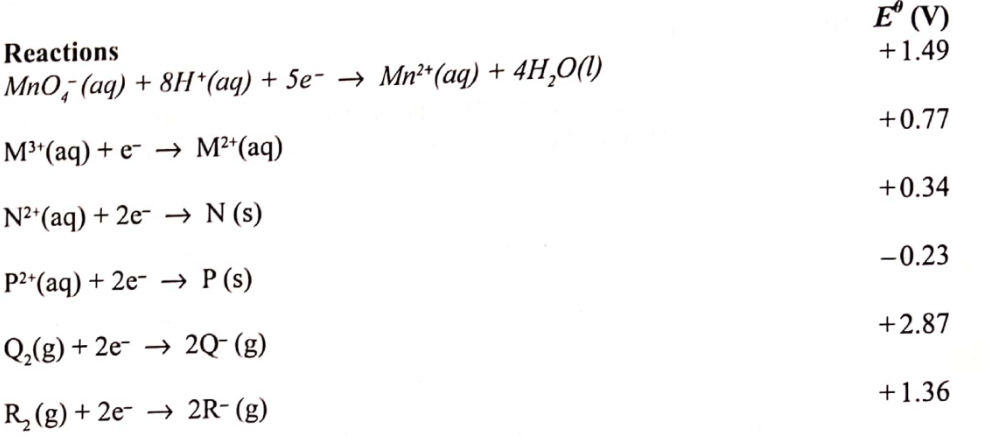
(i) State whether acidified MnO–4 can oxidise M2+. Give a reason. (2 marks)
MNO4will oxidize M2+
Because it has high Eθ value
(ii) Select two half-cells which when combined will give the highest e.m.f. (1 mark)P and Q
(iii) Write the cell representation for the cell formed in b (ii). (1 mark)
Ps/Paq //Q2Q
(iv) Calculate the Eθ value for the cell formed in b (iii). (1 mark)
Eθ value = 2.87 + 0.23
=3.10v
(c) A mass of 1.24g of a divalent metal was deposited when a current of 6A was passed through a solution of a metal sulphate for 12 minutes.Determine the relative atomic mass of the metal( Faraday = 96,500 C mol -1 (3 mark)
Quantity of electricity = 6 x 12 x 60 — 4320 C
2 x 96500 1 mole of metal
4320 C 1.24g of metal
2 x 96500 1.24/4320 x 2 x 96500
55.4
(d) State two application s of electrolysis. (I mark) Decoration Purification of metals Extraction of metals Manufacture of sodium hydroxide
7. (a) What is meant by rate of reaction (1 mark)
Rate = change in concentration
reagents/products/time
Reaction rate is a measure of how much of reactants are consumed or how much products are formed per unit time.
(b) In the space provided, Sketch the diagram of a set-up that Can be used to determine the rate of reaction between manganese(IV) oxide and hydrogen peroxide. (3 marks)
(c) A student placed a small amount of liquid bromine at the bottom of a sealed gas jar of air as shown in Figure 4.

(i) Describe what will be observed: (1 mark)
After two minutes, red/brown colour fumes of bromine are observed on the surface of the bromine liquid.
II. After 30 minutes the gas jar is filled with yellow fumes
(ii) Use the Kinetic theory to explain the observations: (2 marks)Bromine liquid is volatile, the molecules escape from the liquid but the vapour being denser than air will occupy the space on the surface of the liquid.With time, since molecules in the vapour phase move randomly, bromine molecules will undergo collisions with air molecules and mix thus filling the gas jar
(d) Some plants have seeds that contain vegetable oil.
(i) Describe how the oil can be obtained from the seeds. (3 marks)The seeds are crushed in a mortar with a pestle to increase surface area for oil extraction. Then a solvent (ethanol/acetone) is added with continued crushing. The liquid is then decanted into an evaporating dish.
The evaporating dish is placed out into the sun to allow solvent to evaporate because of its low RMM, leaving behind the oil.
(ii) Explain how it could be confirmed that the liquid obtained from the seeds is oil. (1 mark)The liquid left after evaporation is placed on a piece of paper. If it leaves a translucent mark then it proves it is oil

