2011 KCSE General Science Past Paper
2.4.1 General Science Paper 1 (237/1)
SECTION A: BIOLOGY (34 marks)
Answer all the questions in the spaces provided.
1 (a) Name the branch of biology that deals with the study of animals. (1 mark)
(b) Give two reasons for classifying living organisms. (2 marks)
(c) Give a reason why respiration is important in living organisms. ( 1 mark)
2 (a) Name the organelles observed under the light microscope in plant cells but not in animal cells. (2 marks)
3 In an experiment a solution was poured around a potted plant and left for 24 hours. The set up is as shown below.
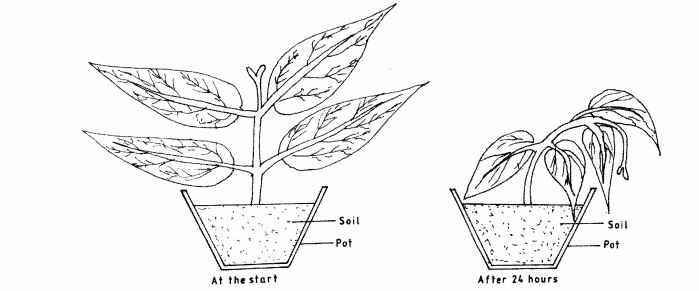
(a) State the nature of the solution. (1 mark)
(b) Explain the observations made after the 24 hours. (2 marks)
4 (a) Name one raw material for photosynthesis. (1 marks)
(b) With the exception of guard cells, how are the upper epidermal cells of a leaf adapted to their function? (2 marks)
5. (a) Distinguish between ingestion and egestion (1 mark)
(b) The diagram below represents a Villus of the human alimentary canal.
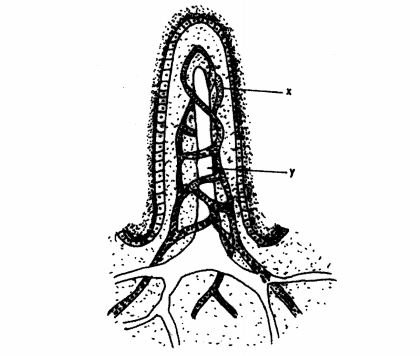
Name the substances that are absorbed through the structures labelled X and Y. (2 marks) X: ……………………………
Y: ……………………………
6. Explain why a person in a poorly ventilate room with a burning charcoal stove may suffocate to death.(3 marks)
7. (a) Name the causative agent of pneumonia.
(b) State two characteristics of an efficient respiratory surface which are absent in an amoeba.(2 marks)
(c) How is anaerobic respiration applied in the baking industry?(1 mark)
8 (a) Explain how euphorbia leaves affect its transpiration.(2 marks)
(b) Explain one adaptation of the root hair to its function.(2 marks)
9 (a) State two functions of the kidney.(2 marks)
(b) Name two metabolic wastes removed through the skin.(2 marks)
10 State the effects of vasodilation.(2 marks)
SECTION B : CHEMISTRY (33 marks)
Answer all the questions in this section in the spaces provided.
11 What is meant by the following terms
Valency. (1 mark)
Electron affinity. (1 mark)
12 The information in the table below gives atomic sizes of elements P, Q and R that belong to the same group in the periodic table. Study it and answer the question that follows. P, Q and R are not the actual symbols of the elements.
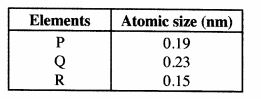
Which element has the highest ionisation energy? Explain. (2 marks)
13 (a) Why is air considered a mixture and not a compound? (1 mark)
(b) Give one similarity between rusting and combustion. (l mark)
(c) Which reagent will decompose to give oxygen gas in the presence of manganese (lV) oxide? (1 mark)
(d) Carbon (IV) oxide does not support buming yet a piece of burning magnesium ribbon continues to bum in a gas jar full of carbon (IV) oxide. Explain. (1 mark)
14 (a) Using dots(-) and crosses (x) to represent electrons. draw the structure of sodium chloride. (Na = 11.0. Cl = 17.0). (2 marks)
(b) Name the type of bond in diamond. (1 mark)
15 Describe how a student would obtain sand from a mixture of sand and sugar. (2 marks)
16 (a) State the purpose of PH scale. (1 mark)
(b) Hydrochloric acid is a strong acid. Explain the meaning of a strong acid. (1 mark)
(c) Dilute hydrochloric acid was reacted with solid calcium carbonate in a test tube. Write a balanced chemical equation for the reaction. (1 mark)
(cl) Give two disadvantages of washing clothes in hard water using soapy detergents. (2 marks)
17 (a) Describe a test that can be used to show presence of water in a substance. (2 marks)
(b) Magnesium ribbon reacts slowly with water to form a solution that turns red litmus paper blue. Name the solution formed. (1 mark)
18 State how the following substances conduct electricity.
(a) Molten calcium chloride. (1 mark)
(b) Graphite. (1 mark)
19 The diagram below shows some changes in the physical states of matter. Study it and answer the questions that follows.

(a) Name the changes represented by letters R and S. (2 marks)
R: ……………………
S: …………………….
(b) Name the method used to separate coloured substances in a dye. (1 mark)
20 The grid below represents part of the periodic table. Use it to answer the questions that follow.
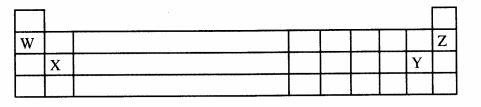
The letters are not the actual symbols of the elements.
(a) Which letter represents an alkaline earth metal? (1 mark)
(b) Which of the two elements represented by the letters W and Z is reactive. Explain. (2 marks)
(c) Write the electron arrangement of the element represented by Y. (1 mark)
21 (a) Name the type of reaction that occurs when a solution of lead (II) nitrate is added to a solution of sodium sulphate (in a boiling tube). (1 mark)
(b) Write a balanced equation for the reaction that occurs when crystals of sodium nitrate are heated in a test tube. (1 mark)
(c) Give the meaning of an acid salt. (1 mark)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this section in the spaces provided.
22 Figure 1, shows a measuring instrument containing a liquid.
(a) State the name of the instrument. (1 mark)
(b) The reading changes from 26.8cm3 to 42.5cm’ after 40 drops of the liquid are released, determine the volume of each drop. ‘ (2 marks)
23 (a) A block of wood is pulled alon% a horizontal surface. State one factor that determines the magnitude of the frictional orce between the block and the surface. (1 mark)
(b) Figure 2 shows a vertical glass tube containing a liquid.
State a reason for the shape of the meniscus in terms of molecular forces. (2 marks)
24 (a) Figure 3 shows two identical containers A and B filled with different liquids to the same level
Figure 3
It is observed that the pressure at the bottom of container B is higher than the pressure at the bottom of container A. State the reason for this observation. (1 mark)
(b) Smoke particles inside a smoke cell are observed to move randomly when viewed through a microscope. Explain this observation. (2 marks)
25 A student observes that in the morning an overhead electrical power cable is straight _ and taut. At midday the student observes that the same cable has sagged. Explain these observations (2 marks)
26 Figure 4, shows a crystal of potassium permanganate at the bottom of a beaker containing some water.
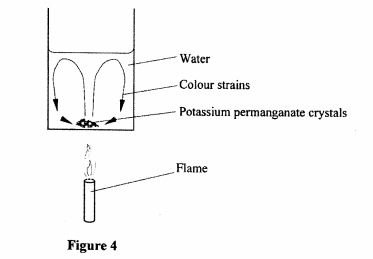
Figure 4
It is observed that when the beaker is heated from the bottom, strains of colour rise up from the crystal and curve out as shown. Explain this observation. (3 marks)
27 Figure 5, shows a uniform meter rule pivoted at the 60cm mark. The rule is balanced when a 40g mass is supported at the 90cm mark.
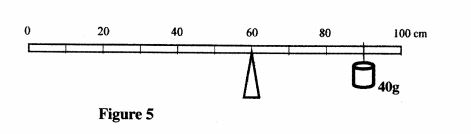
Figure 5
(a) Show on the diagram the position of the centre of gravity of the metre rule. (1 mark)
(b) Determine the mass of the metre rule. (2 marks)
28 Figure 6, shows a ball bearing resting on a flat surface.
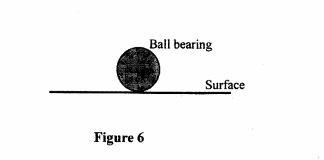
Figure 6
(a) Name the state of equilibrium of the ball bearing. (1 mark)
(b) State the reason for the answer in (a) above. (2 marks)
29 A spring extends by 20mm when supporting amass of 150g. Determine the mass which when supported by the same spring causes an extension of 30mm. (3 marks)
30 A stone is thrown vertically upwards. Sketch a velocity – time graph for the motion of the stone from the time it is thrown until it comes back to the ground. (2 marks)
31 A box is lying on the floor of a fast moving lorry, it is observed that when the lorry is suddenly brought to rest, the final position of the box is nearer to the front of the lorry than before. Explain this observation. (2 marks)
32 Figure 7, shows a pully being used to raise a load.
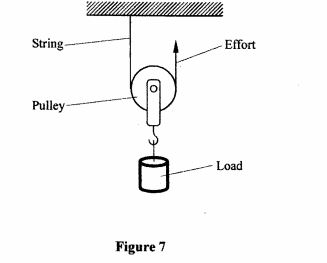
Figure 7
State the velocity ratio of this machine. (1 mark)
(b) State two factors that reduce the efficiency of this machine. (2 marks)
33 (a) State the law of flotation. (1 mark)
(b) A ship is made of steel. Explain why the ship is able to float on water whose density
is less than the density of steel. (2 marks)
2.4..2 General Science Paper 2 (237/2)
SECTION A: BIOLOGY (34 marks)
Answer all the questions in the spaces provided.
1 (a) Name two abiotic factors in a soil ecosystem.(2 mark)
(b) Give an example of parasitism in wood-land ecosystem.(2 mark)
2 (a) State two reasons for sexual reproduction in animals.(1 mark)
(b) Distinguish between fertilization and ovulation.(1 mark)
(c) Name the hormone that is responsible for the growth of beards.(1 mark)
3 (a) State one role played by both bacteria and fungi in nitrogen cycle.(1 mark)
(b) Give one adaptation of hydrophyte roots.(1 mark)
(c) Name a method that can be used to control air pollutants from a factory.(1 mark)
4 The graph below illustrates growth in an animal.
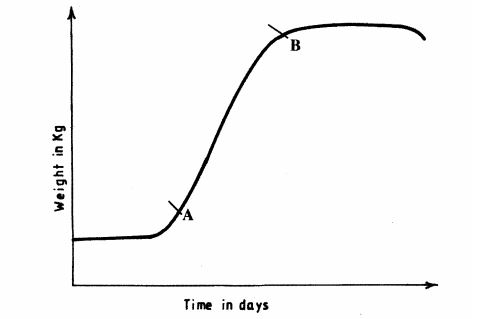
(a) Explain what happens between points A and B.(1 mark)
(b) What is meant by the term seed dormancy?(1 mark)
(c) Explain what happens when the shoot apex of a plant is removed.(2 mark)
5 (a) Explain how the sex of a child is determined by a man.
(b) A heterozygous red-eyed fruit fly was crossed with a recessive white-eyed one. Using ‘R’ to represent the dominant gene, work out the cross to show the offspring. (3 marks)
(c) State the phenotypic ratio of the offspring. (1 mark)
6 State two ways in which sexually transmitted infections can be avoided. (2 marks)
7 Explain the importance of strictly following the prescription given when taking medicine. (2 marks)
8 (a) Name two supportive tissues in dicotyledonous plants. (1 marks)
(b) Give an example of a hinge joint in humans. (1 mark)
9 (a) State two differences between the endocrine and the nervous systems. (2 marks)
(b) State one function for each of the following structures in the mammalian ear. (1) Pinna (1 mark)
(ii) ossicles (1 mark)
10 The diagram below represents a type of neurone found in animals.
(a) Name the neurone. (1 mark)
(b) Give the reason for your answer in (a) above. (1 mark)
SECTION B: CHEMISTRY (33 marks)
Answer all the questions in this section in the spaces provided.
11 Under the same conditions of temperature, volume and pressure, which one of the following gases; steam, carbon(IV) oxide, nitrogen, oxygen and ammonia would diffuse the slowest? Explain. (R.A.M: H = l, C =12, N =14 and 0 =16). (2 marks)
12 Hydrogen combines with oxygen to form water. How many moles of hydrogen atoms does 3.6g of water contain? (2 marks)
(H = 1, O = 16)
13 One of the methods used for large scale production of ethanol is by fermentation of cane sugar.
(a) What is meant by fermentation‘? (2 marks)
(b) The ethanol obtained is about 4 – 8% concentrated. How can this concentration be increased? (1 mark)
(c) Other than being used as an alcoholic beverage, state one use of ethanol. (1 mark)
14 Dilute hydrochloric acid was reacted with magnesium ribbon and the volume of hydrogen gas evolved was measured at 10 seconds interval up to 60 seconds. The volumes were recorded as in the table below.
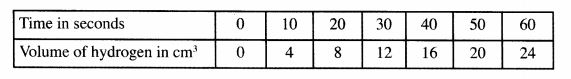
(a) Using the grid provided draw a graph of volume of hydrogen (vertical axis) against time (horizontal axis). (3 marks)
Using the graph, determine the average rate of production of hydrogen. (1 mark)
15 In the manufacture of ammonia using the haber process, nitrogen and hydrogen gases react as shown in the equation below.
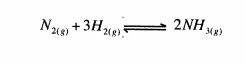
(a) State the source of hydrogen gas.(1 mark)
(b) Explain the effect of increasing pressure on the yield of ammonia. (2 marks)
(c) Give one use of nitrogen (IV) oxide.(1 mark)
16 The diagram below represents electrolytic production of aluminium metal. Study it and answer the questions that follows.
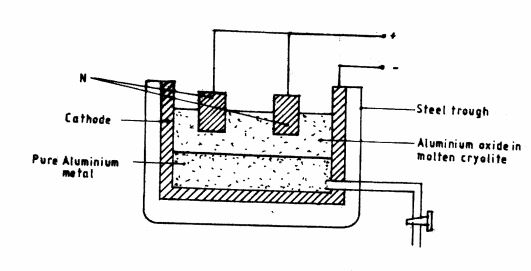
(a) Why is aluminium extracted by electrolysis and not reduction? (1 mark)
(b) Why is N replaced regularly?(1 mark)
(c) State one use of the molten cryolite in the above process.(1 mark)
(d) State two properties that makes aluminium metal to be widely used in electric cables. 17 (a) What is meant by the term molar solution?(2 marks)
(b) Calculate the molarity of a solution containing 6.24g of hydrated copper (ll) sulphate crystals in 250cm-‘ of solution. (R.F.M CuSO4.5H_2O = 249.5). ( 2 marks)
18 The energy level diagrams below shows the enthalpy changes for reactions A and B.
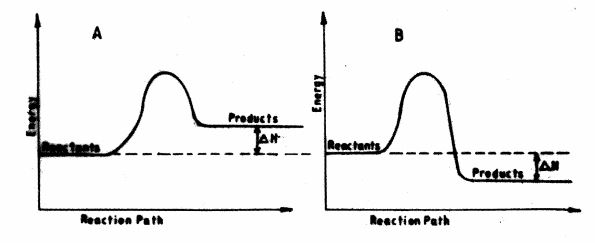
(a) State with a reason the type of reactions represented by A and B. (2 marks)
A ……………………….
B ……………………….
(b) State two properties of carbon (XV) oxide that make it qualify to be used as a fire extinguisher. (2 marks)
(c) Give one advantage of using biogas as a fuel instead of firewood. (1 mark)
19 State Graham’s law of diffusion. (1 mark)
20 Chlorine gas is collected using the method shown in the figure below.
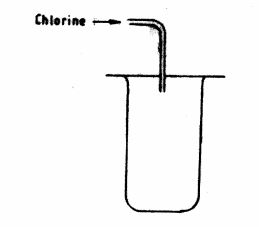
(a) Name the method of collection. (1 mark)
(b) Which property of chlorine enables it to be collected using the method shown above? (1 mark)
(c) Give two uses of chlorine gas. (1 mark)
SECTION C – PHYSICS (33 marks)
Answer ALL the questions in this section in the spaces provided.
21 (a) Figure 1 shows a ray of light incident on a converging mirror through the centre of a curvature C.
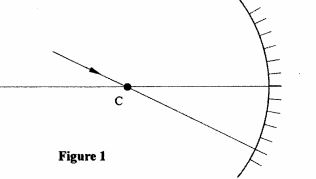
Figure 1
Complete the diagram to show the reflected ray. (1 mark)
22 Figure 2 shows two rays from a point object O incident on a plane mirror

Figure 2
Complete the diagram to show the position of the image. (2 marks)
23 A plastic ruler becomes negatively charged when rubbed with a piece of cloth. Explain how the ruler acquires the charge. (2 marks)
24 Given a voltmeter, an ammeter, a resistor, a switch and a cell. draw a circuit diagram that may be used to measure the voltage across the resistor and the current through it. (2 marks)
25 Draw a labelled diagram showing how two bar magnets should be stored using keepers.
(1 mark)
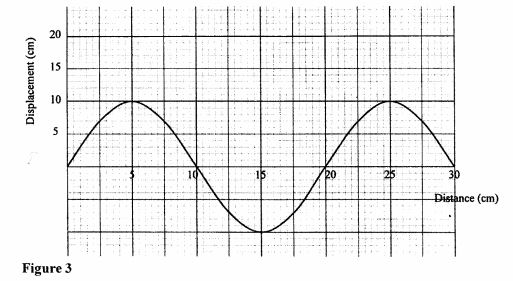
Figure 3
Determine:
(a) the amplitude of the wave, (1 mark)
(b) the Wavelength of the wave. (1 mark)
27 A girl standing in front of a wall claps her hands. She hears the echo 0.4 seconds later.
Determine the distance between the girl and the wall.
(take speed of sound in air to be 340ms”) (3 marks)
28 Figure 4 shows the face of an ammeter indicating a current.
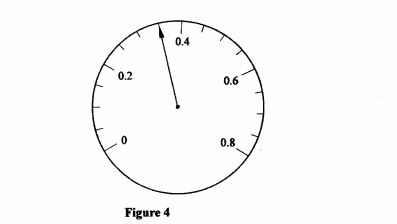
Figure 4
State the value of the current indicated. (1 mark)
29 Figure 5 shows a ray of light travelling from air to glass.
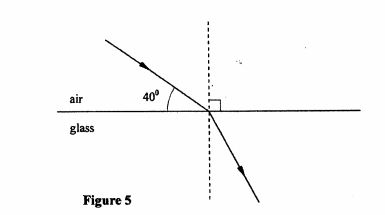
Figure 5
(a) Indicate on the diagram the angle of incidence, of the ray. (1 mark)
(b) Given that the refractive index for a ray travelling from air to glass is l .5, determine ‘ the angle of refraction. (2 marks)
30 Figure 6 shows an object O placed in front of a converging lens whose principal focus is F.
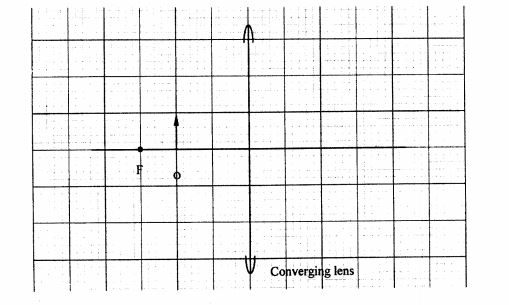
Draw a ray diagram to locate the image formed. p (3 marks)
31 An electric cooker has a resistance of 2052. Determine the power it dissipates when connected to a 240V mains supply. (3 marks)
32 (a) State the purpose of creating a vacuum in a cathode ray tube. (1 mark)
(b) Figure 7 shows a horizontal cathode ray entering an electric field between two charged plates.
Figure 7
Complete the diagram to show the path of the ray in the electric field. (1 mark)
33 State how the produced X-rays change, when the anode potential of the X-ray tube is increased. (1 mark)
34 Name two medical uses of radioactive radiations. (2 marks)
35 (a) State one difference between a semiconductor and a conductor. (1 mark)
(b) Draw a circuit diagram to show a diode connected in the reverse bias mode (1 mark)
36 Figure 8 shows a graph of mass against time for a radioactive sample. mass (g)
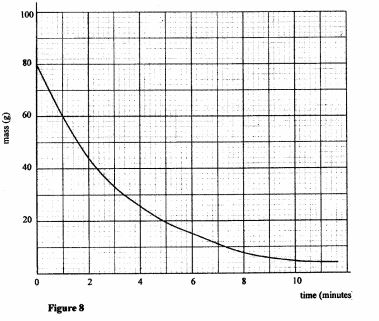
Figure 8 Use the graph to determine the half life of the sample. (2 marks)
2011 KCSE General Science Past Paper-Marking Scheme/Answers
1 (a) Zoology; (1 marks)
(b) To sort and group organisms;
For uniformity in identification;
Organise information in an orderly manner to avoid chaos;
Put Organisms into correct groups to make their study easy.(2 mark)
(c) Production of energy; I (1 mark)
2 (a) Cell wall; chloroplasts; sap vacuole (2 mark)
(b) Hold the microscope firmly with both hands
(one hand at the arrniand the other at the base);
Place the microscope away from the edge of the table.
(2 mark)
3 (a) Hypertonic; (1 marks)
Solution of high concentration.
(b) Through osmosis, the plant lost water to the soil environment; this caused the plant to droop; (2 marks)
Plant cells lost water -H20 to the high concentration;
Through osmosis cells flaccid leading to dropping/witting.
4 (a) Carbon (IV) Oxide; water; (any one correct) (1 maks)
(b) Closely packed to protect inner cells;
Lack of chloroplasts/transparent to allow light to pass through; the cells are one layer thick/thin in diameter to allow light to penetrate easily;
(any 2 correct) (2 marks)
5 (a) Ingestion is taking in food material through the mouth while egestion is the removal of undigested/indigestible food materials thought the anal opening; (1 mark)
(b) (i) in X glucose/amino acids/vitamins; (1 mark)
(ii) in Y fatty acids/glycerol. (1 mark)
6 Burning charcoal stove reduces the amount of oxygen in the room leading to partial burning; which produce carbon(II) oxide; when inhaled it combines permanently with haemoglobin/blocking uptake of oxygen; I (3 marks)
7 (a) Pneumococcus/Diplococcus pneumoniae/streptococcus pneumonial/stephilococcus auries; (1 mark)
(b) High vascularization; ventilation mechanism; (2 marks)
(c) Produce carbon (IV) oxide that raises dough; (1 mark)
8 (a) Leaves are tiny/small; reducing the surface area over which transpiration occurs/rate of transpiration is reduced;
Thick, shinny cuticle; reduce rate of transpiration; (2 marks)
(b) Elongated cells; to provide a large surface area for nutrients/water absorption;/Thin; to facilitates diffusion of substances; (2 marks)
9 (a) Excretion; osmoregulation;
PH regulation;
Ionic balance.
(2 marks)
(b) Water; salts; (2 marks)
10 More blood flows closer to the skin;
More heat is lost cooling occurs; (2 marks)
CHEMISTRY – SECTION B – (33 marks)
11 Valency – combining power of an element/radical; or number of electron gained or lost by an atom (1 mark)
Electron affinity – energy released when an atom acquires an electron; (1 mark)
12 R; R is the smallest atom with its outermost electrons near the nucleus hence strongly attracted;
(2 marks)
13 (a) Gases in air are separated by physical means;
(b) Both require oxygen gas;
(c) Hydrogen peroxide;
(d) Magnesium has high affinity for the combined oxygen;heat produced by burning magnesium, decomposes carbon (VI) oxide to carbon and oxygen. (4 marks)
14. (a)
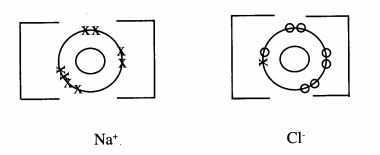
(b) Covalent bond; (1 mark)
15. Add Water to the mixture, warm and stir, sugar will dissolve;
Filter the mixture to obtain sand as a residue; (2 marks)
16. (a) To measure the acidity and alkalinity of a solution;/measure the strengths of acids and Bases.
(b) Strong acid is one that dissociates fully to give more hydrogen ions;
(c) 2HCl + CaCo3($) ‘- CaC|2(ao) + Cong + HzO(|);
(d) Waste of soap;/not economical
formation of scum stains on clothes (5 marks)
17. (a) Use anhydrous Copper (II) Sulphate; if it changes form white to blue when in contact with the substance then water is present; /blue anhydrous cobalt chloride paper of cob: sulphate to pink
(b) Magnesium hydroxide; =(3 marks)
18. (a) Mobile ions;
(b) Delocalised electrons; (2 marks)
19. (a) R – freezing/solidifying;
S – Condensation/liquefying;
(b) Chromatography;/paper chromatography (3 marks)
20. X;
(b) W; W is an alkali metal
Z is a noble element which is stable; Since it has an octet structure or is in grou VIII
(c) 2.8.7; (4 marks)
21. (a) double decomposition/precipitation;
(b) 2NaNO3(s) 2NaNO2(s) + 0219;;
(c) Salt that contains replaceable hydrogen atoms; (3 marks)
PHYSICS – SECTION C – (33 marks)
22. (a) (b)Burette (1 mark)
(l mfll Vol of 40 drops = 42.5 – 26.8 = 15.7cm3 Vol of l drop =li1 = 0.39 40 = 0.4cm3 (2 mat 364
23 (a) Nature of the surface/roughness/smoothness; Normal reaction. (l mark)
(b) Attraction forces between molecules of the liquid is less than the attraction of the liquid molecules and glass molecules/adhesive forces are greater than cohesive forces. (2 marks)
24 (a) Density of B is greater than that of A. (1 mark)
(b) Smoke particles are hit by air molecules which are moving in 21 (continuous) random motion.(2 marks)
25 In the morning air is cold and the cable contracts becoming shorter.
At midday the air is hot and the cable expands becoming longer hence sags. (2 marks)
26 When heated the water molecules expand and become less dense.
They rise up and cooler more dense water molecules move downwards. (2 marks)
The potassium paramagnet color moves up with the less dense molecules hence the colour strains curve up as the water moves in convectional currents. (1 mark)
27 (a) at 50cm mark/or on dieagram.
(l mark)
(b)

28 (a) neutral (1 mark)
(b) When the ball bearing is slightly tilted, the position of centre of gravity remains unchanged. (2 marks)
29 imagee equation
30

31. Before the brakes are applied the box was moving at the same speed as the lorry. It continues moving forward due to its inertia when the brakes are suddenly ~. applied/obeys NeWton’s first law of motion..
(2 marks)
32. (a) 2. (1 mark)
(b) – weight of the pulley
– amount of friction between the pulley and the string. (2 marks)
33. (a) A floating body displaces its own weight of the fluid in which it floats. (1 mark)
(b) The ship is “hollow” and therefore it’s average density is less than that of water.
(2 mark)
12..2 General Science Paper 2 (237/2)
BIOLOGY
SECTION A: (34 marks)
1 (a) Air; moisture;salinity; P“; temperature; any two. (2 x 1 ) (2 marks)
(b) Ticks on buffaloes/tse-tse flies on water bucks fleas on monkeys; accept any other correct relationship. (l X 2) (2 marks)
2 (a) Pass hereditary characteristics to future generations;
Perpetuate the species/survival;
Continuation of life
Natural selection/enhances variations; any two. _ (2 x 1 ) (2 marks)
(b) Fertilization – fusion of sperm and egg to form zygote while ovulation is the release of the ovum from the ovary into the fallopian tube; (mark as a whole) ~
(1 mark)
(c) Testosterone; (1 mark)
3 (a) Decomposition/decay; (1 mark)
(b) Long/fibrous roots; for anchorage in /absorption of nutrients from Water. (1 mark)
(c) By converting pollutants to harmless substances; accept recycling. (1 mark)
4 (a)Fast/rapid/exponential growth; many cells are dividing/optimum environmental conditions; (2 marks)
(b) A period during which a viable seed undergoes no growth; (1 mark)
(c) Lateral buds sprout; due to reduced supply of auxins; (2 marks)
(d) A period during which a seed does not germinate even if in favourable conditions.
5 (a) The man produces two types of sperms one containing X chromosomes and the other Y chromosomes; while the woman produces ova with only X chromosomes; If the X sperm fenilizes the ovum the result is a girl and if the Y sperm fertilizes the ovum the result is a boy; (maximum two marks). (2 marks)
(b)


