2013 KCSE Chemistry Past Paper
4.5.1 Chemistry Paper 1 (233/1)
1. The set up below can be used to prepare oxygen gas. Study it and answer the questions that follow.
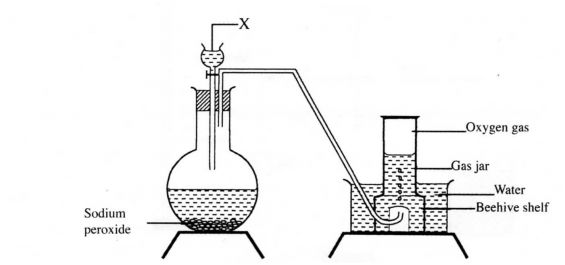
(a) Identify gas x ? (b) What property of oxygen makes it possible for it to be collected as shown in the above set up? (1mark)
(c) State two uses of oxygen. (1mark)
2 Write equations to show the effect of heat on each of the following: (a) sodium hydrogen carbonate; (1mark)
(b) silver nitrate; (1 mark)
(c) anhydrous iron (ll) sulphate. (1 mark)
3 Describe an experimental procedure that can be used to extract oil from nut seeds. (2 marks)
4 In terms of structure and bonding, explain the following observations: (a) the melting point of aluminium is higher than that of sodium: (1 /2 marks)
(b) melting point of chlorine is lower than that of sulphur. (1 % marks)
5.The diagram below illustrates a method of preparing salts by direct synthesis.
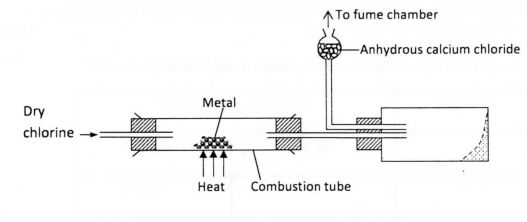
(a) This method can be used to prepare either aluminium chloride or iron (III) chloride. Explain why it cannot be used to prepare sodium chloride. (1 mark)
(b) Describe how a sample of sodium chloride can be prepared in the laboratory by direct synthesis. (2 marks)
6.(a) A student electroplated a spoon with copper metal. Write an equation for the process that took place at the cathode. (1 mark)
(b) Calculate the time in minutes required to deposit 1.184g of copper if a current of 2 amperes was used. (1 Faraday = 96500 coulombs, Cu = 63 .5). (2 marks)
7.Study the flow chart below and answer the questions that follow:
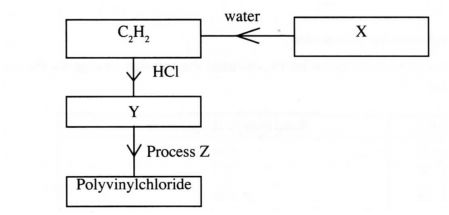
(a) Identify:
(i) X (1mark)
(ii) Y (1 mark)
(b) State two uses of polyvinylchloride. (1 mark)
8.Draw a labelled diagram to illustrate how alpha, beta and gamma radiations can be distinguished from each other. (3 marks)
9.Aqueous hydrogen chloride reacts with potassium manganate (VII) to produce chlorine gas, while a solution of hydrogen chloride in methylbenzene has no effect on potassium manganate (VII). Explain this observation. (2 marks)
10.The table below gives the solubilities of substances T and U at 10°C and 40°C. Substance Solubility g/100g water
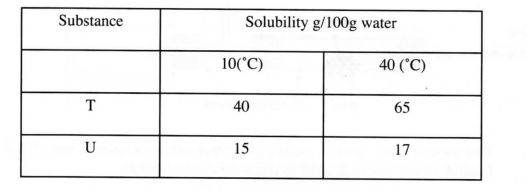
When an aqueous mixture containing 55g of T and 12g of U at 80°C was cooled to 10°C, crystals formed.
(a) Identify the crystals formed. (1 mark)
(b) Determine the mass of the crystals formed. (1 mark)
(c) Name the method used to obtain the crystals. (1 mark)
11.Hydrazine gas,
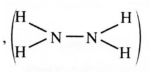
burns in oxygen to form nitrogen gas and steam.
(a) Write an equation for the reaction. (1 mark)
(b) Using the bond energies given below. calculate the enthalpy change for the reaction in (a) above. (2 marks
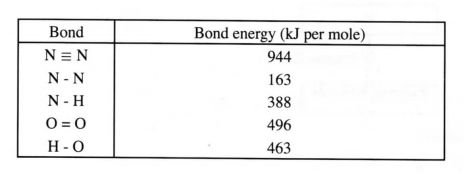
Bond Bond energy (k/ per mole)
12 (a) What would be observed if sulphur (IV) oxide is bubbled through acidified potassium manganate (VII)? (1 mark)
(b) In an experiment, sulphur (IV) oxide was dissolved in water to form solution L.
(i) What would be observed if a few drops of barium nitrate solution were immediately added to solution L? (1 mark)
(ii) Write an ionic equation for the reaction that occurred between solution L and aqueous barium nitrate in (b)(i) above. (1mark)
13 The scheme below shows some reaction sequence starting with solid N. Study it and answer
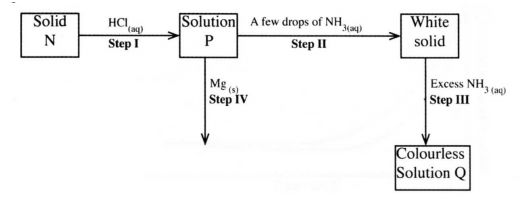
(a) Write the formula of the complex ion in solution Q. (1mark)
(b) Write an equation for the reaction in step IV. (1 mark)
14 (a) State the Charles’ law. (1 mark)
(b) A certain mass of gas occupies 146 dm’ at 291 K and 98.31 kPa.
What will be its temperature if its volume is reduced to 133 dm’ at 101.325 kPa? (2 marks)
15 The chromatogram below was obtained from a contaminated food sample P. Contaminants
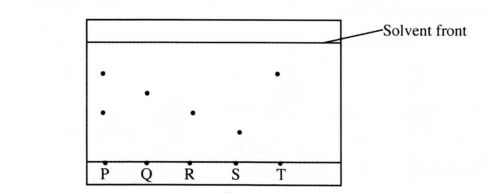
Q, R, S and T are suspected to be in P. Use it to answer the following questions. Solvent front
P Q R S T (b) Which is the most soluble contaminant in P? (1 mark)
16 The curves below represent the change in mass when equal masses of powdered zinc and zinc granules were reacted with excess 2M hydrochloric acid. Study them and answer the question below.
Which curve represents the reaction with zinc granules? Explain your answer. (3 marks)
17 When fuels bum in the intemal combustion engine at high temperature, one of the products formed is nitrogen(ll) oxide.
(a) Write the equation for the formation of nitrogen(ll) oxide. (1 mark)
(b) Give a reason why nitrogen(ll) oxide is not formed at room temperature. (1 mark)
(c) Describe how formation of nitrogen (II) oxide in the internal combustion engine leads to gaseous pollution. (1 mark)
18 The set-up below was used to investigate the products of buming biogas (methane). Study it and answer the questions that follow.
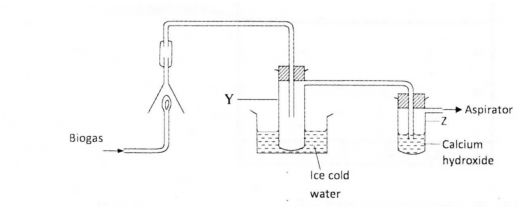
(a) What product will be formed in test-tube Y? (1 mark)
(b) State and explain the observations which would be made in Z. (2 marks)
19 (a) Diamond and graphite are allotropes of carbon. What is meant by an allotrope? (1 mark)
(b) Explain why graphite can be used as a lubricant while diamond cannot. (2 marks)
20.The plots below were obtained when the atomic radii of some elements in groups I and II were plotted against atomic numbers.
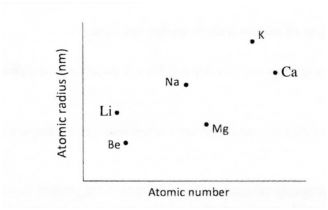
Explain:
a)the trend shown by Li, Na and K. (1 mark) b)why the atomic radii of elements Be, Mg and Ca are lower than those of Li, Na and K. (2 marks)
21. On heating a pale green solid K, carbon (IV) oxide gas and a black solid M were formed. On reacting K with dilute hydrochloric acid, carbon (IV) oxide gas and a green solution S were formed. When excess aqueous ammonia was added to solution S. a deep blue solution was formed.
a)Identify the cation in solid K. (1 mark)
b)Identify the two anions in solution S. (2 marks)
22. a) Name two ores from which copper is extracted. (1mark)
b)During extraction of copper metal, the ore is subjected to froth flotation. Give a reason why this process is necessary. (1mark)
c c)Name one alloy of copper and state its use. (1mark)
Alloy
Use
23. When l5cm3 of a gaseous hydrocarbon, P, was burnt in 100cm-‘ of oxygen, the resulting gaseous mixture occupied 70cm-‘ at room temperature and pressure. When the gaseous mixture was passed through potassium hydroxide solution, its volume decreased to 25cm’.
a)What volume of oxygen was used during the reaction? (1mark)
b)Determine the molecular formular of the hydrocarbon. (2 marks)
24. A solution was made by dissolving 8.2g of calcium nitrate to give 2 litres of solution. (Ca = 40.0: N = 14.0; O = 16.0).
Determine the concentration of nitrate ions in moles per litre. (3 marks)
25) State and explain what would happen if a dry red litmus paper was dropped in a gas jar of dry chlorine. ( 2 marks)
26. By using aqueous sodium chloride. describe how a student can distinguish calcium ions from lead ions. ‘ (2 marks)
27. A student investigated a property of acids M and N by reacting equal volumes of acid M and N of the same concentration with equal volumes of 2M potassium hydroxide. The results were recorded in the table below.
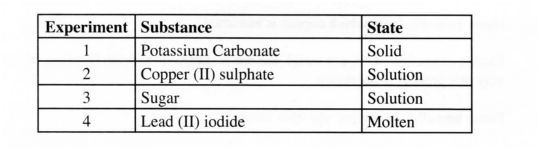
(a) Which of the acids is likely to be a weak acid? Explain. (2 marks)
(b) Write the equation for the reaction between ethanoic acid and potassium hydroxide. (1 mark)
28. A student investigated the effect of an electric current by passing it through some substances.

The student used inert electrodes. and connected a bulb to the circuit. The table below shows the substances used and their states.
Experiment: Substance State
1:Potassium Carbonate Solid
2 :Copper (II) sulphate Solution
:Sugar Solution
:Lead (II) iodide Molten
(a) In which experiments did the bulb not light? (1 mark)
(b) Explain your answer in (a) above. (2 marks)
29. A sample of hydrogen gas was found to be a mixture of two isotopes, ‘1-1and IH. I I Determine the relative molecular masses of the molecules formed. when each of these isotopes is burnt in oxygen. (O = l6.0)
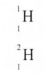
4.5.2 Chemistry Paper 2 (233/2)
1 )The grid given below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements. M N P T R

a)(i) Select a letter which represents an element that loses electrons most readily.
Give a reason for your answer. (2 marks)
(ii) Explain why the atomic radius of P is found to be smaller than that of N. (2 marks)
(iii) Element M reacts with water at room temperature to produce 0.2 dm~‘ of gas. Determine the mass of M which was reacted with water. (Molar gas volume at room temperature is 24 dma, Relative atomic mass of M = 7). (3 marks)
b) Use the information in the table below to answer the questions that follow. (The letters are not the symbols of the elements)
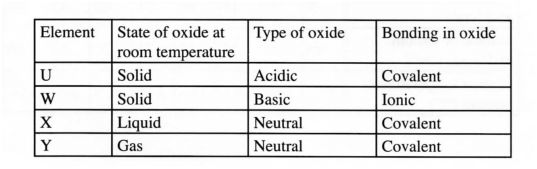
Element. State of oxide at Type of oxide Bonding in oxide room temperature
Solid Acidic Covalent
Solid Basic Ionic
Liquid Neutral Covalent, p> Gas Neutral
Covalent
b)Identify a letter which represents an element in the table that could be calcium, carbon or sulphur. Give a reason in each case.
(i) Calcium:
…………………………………………………………………………………… ..(2 marks)
Reason
………………………………………………………………………………………… ..
(ii) Carbon:
…………………………………………………………………………………….. ..(2 marks)
Reason . . . . . . . . . . . . . . . . . . . . . . . . . . . ..
(iii) Sulphur:
……………………………………………………………………………………. ..(2 marks)
Reason
………………………………………………………………………………………… ..
2 (a) (i) What is meant by the term ‘Enthalpy of formation“? (1 mark) (ii) The enthalpies of combustion of carbon, methane and hydrogen are indicated below:
I0 Draw an energy cycle diagram that links the enthalpy of formation of methane to enthalpies of combustion of carbon, hydrogen and methane. (2 marks)
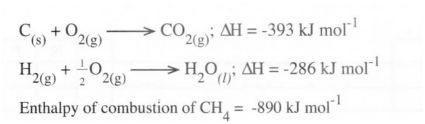
II) Determine the enthalpy of formation of methane. (2 marks)
(b) .An experiment was carried out where different volumes of dilute hydrochloric acid and aqueous sodium hydroxide both at 25°C were mixed and stirred with a thermometer. The highest temperature reached by each mixture was recorded in the table below:
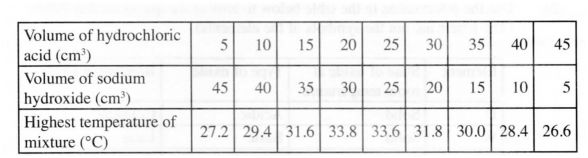
b)On the grid provided, plot a graph of highest temperature (vertical axis) against
(i) Identify X. (ii) Using your graph, detemiine the:
l highest temperature reached;
II volume of acid and base reacting when highest temperature is reached;
(iii) Calculate the amount of heat liberated during the neutralisation process (Specific heat capacity is 4.2 J g‘ K‘ and the density of solutions is 1.0 g cm )
c) The molar enthalpy of neutralisation between hydrochloric acid and ammonia solution was found to be -52.2 kJ mol”, while that of hydrochloric acid and sodium hydroxide was -57.1 kJ mol”. Explain the difference in these values. (2 marks)
3 a) The diagram below shows the Frasch process used for extraction of sulphur. Use it to answer the questions that follow.
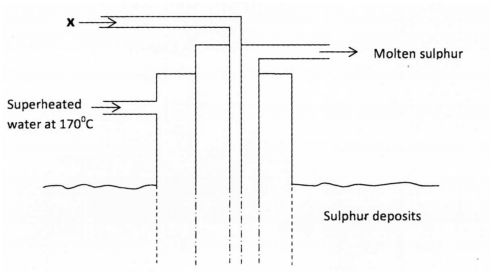
(1 mark)
(ii) Why is it necessary to use super heated water in this process? (1 mark) (iii) State two physical properties of sulphur that makes it possible for it to be extracted by this method. (2 marks)
(b) The diagram below shows part of the processes in the manufacture of sulphuric (VI) acid. Study it and answer the questions that follow.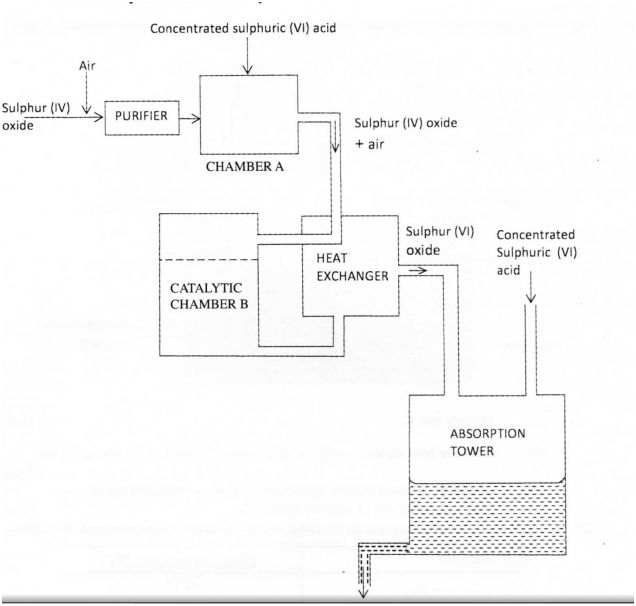 (i) Write an equation for the formation of sulphur (IV) oxide from sulphur.
(i) Write an equation for the formation of sulphur (IV) oxide from sulphur.
(ii) What is the role of concentrated sulphuric (VI) acid in chamber A? (1 mark)
(iii) Name two catalysts that can be used in the catalytic chamber B. (2 marks) (c) Explain one way in which sulphur (IV) oxide is a pollutant. (I mark)
(d) What observation will be made when a few drops of concentrated sulphuric (VI) acid are added to crystals of sugar? Explain your answer. (1 mark)
(4) a) The set up below can be used to produce sodium hydroxide by electrolysing brine.
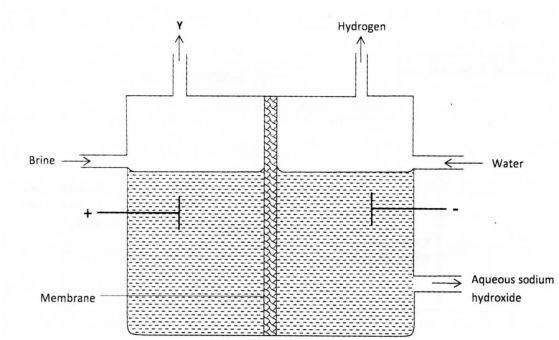
Identify gas Y. (1 mark)
(ii) Describe how aqueous sodium hydroxide is formed in the above set-up.
(iii) One of the uses of sodium hydroxide is in the manufacturing of soaps. (2 marks)
State one other use of sodium hydroxide. (1 mark)
(b) Study the information given rn the table below and answer the questions that follow.

i) Construct an electrochemical cell that will produce the largest emf. (3 marks)
ii)Calculate the emf of the cell constructed in (i) above. (2 marks)
iii) Why is it not advisable to store a solution containing E‘ ions in a container made of H? (2 marks)
5. a) Describe one method that can be used to distinguish between sodium sulphate and sodium hydrogen sulphate. starting with lead metal.
(c) Study the flow chart below and answer the questions that follow:
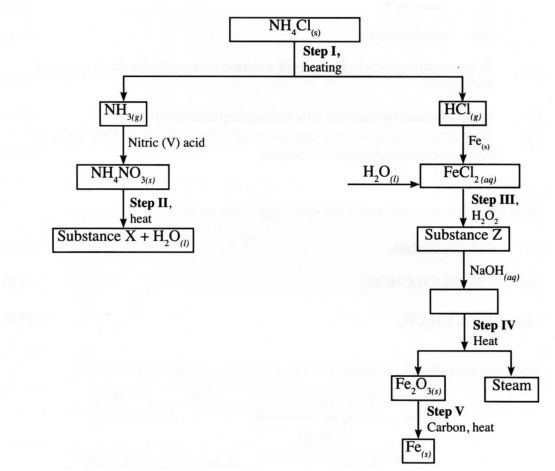
(i) Write an equation for the reaction in:
I step II;
II step IV.
(ii) State the observation made in step IH. Explain.
(iii) Name another substance that can be used in step V.
6. (a) Distinguish between a neutron and a proton.
(b) What is meant by a radioactive substance?
(c) State two dangers associated with radioactive substances in the environment. (2 marks)
(b) Describe how a pure sample of lead (II) sulphate can be prepared in the laboratory
(d) The two isotopes of hydrogen, deuterium (iD) and tritium (iT) react to form element Y and neutron particles, according to the equation below:
(i) What is the atomic:
(I) mass of Y; (l mark)
(II) number of Y. (l mark)
(ii) What name is given to the type of reaction undergone by the isotopes 0f_ hydrogen? (1 mark)
(e) (i) What is meant by half-life of a radioactive substance? (1 mark)
(ii) 288g of a radioactive substance decayed to 9g in 40 days. Determine the half-life of the radioactive substance. (2 marks)
7. (a) Give the systematic names for the following compounds:
(i) Cl-13C]-IZCOOH; (1 mark)
(ii) CH3CH2CH2CHCHZ; (1 mark)
(iii) cn c cnzcna. (1 mark)
(b) Study the flow chart below and use it to answer the questions that follow:
C1 (2)
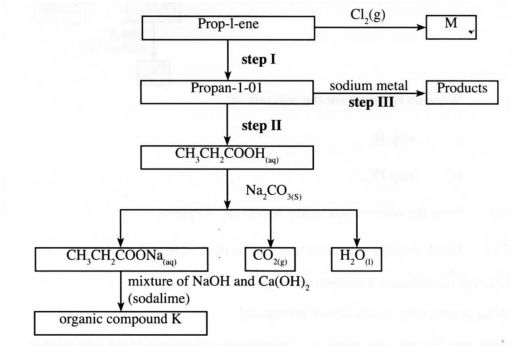
(i) Identify the organic compound K. (l mark)
(ii) Write the formula of M.
(iii) Give one reagent that can be used in:
(I) step I;
(II) step II.
(iv) Write the equation of the reaction in step III.
c) The structure below represents a type of a cleansing agent.
R S05 Na
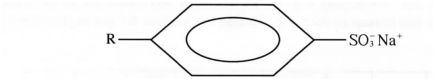
Describe how the cleansing agent removes grease from a piece of cloth. (3 marks)
4.5.3 Chemistry Paper 3 (233/3)
1 You are provided with: solution A’ aqueous copper (H) sulphate; solid B, iron powder; 0.02 M acidified potassium manganate (VII), solution C.
You are required to determine the molar heat of displacement of copper by iron.
Procedure I
Using a burette, place 50.0 cm’ of solution A in a 100 ml beaker. Measure the temperature of the solution and record it in table 1 below. Add all of solid B provided at once and start a‘ stop watch. Stir the mixture thoroughly with the thennometer and record the temperature of the mixture after every one minute in the table. Retain the mixture for use in procedure II below.
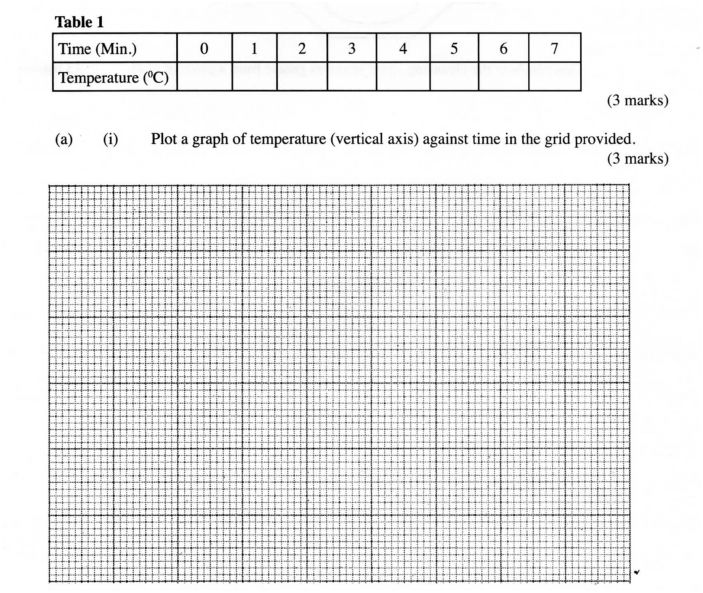
(a) (i) Plot a graph of temperature (vertical axis) against time in the grid provided. (3 marks)
(ii) From the graph, determine the;
(I) highest change in temperature, AT; (1 mark)
(II) time taken for reaction to be completed. (1mark)
(iii) Calculate the heat change for the reaction. (Specific heat capacity of solution is 4.2 Jg” K”; Density of the solution is l gem”). (2 marks)
Procedure II Carefully decant the mixture obtained in procedure I into a 250 ml volumetric flask. Add about 10 cm’ of distilled water to the residue in the 100 ml beaker. Shake well, allow the mixture to settle and carefully decant into the volumetric flask. Immediately, add about 50 cm‘ of 2 M sulphuric (VI) acid to the mixture in the volumetric flask. Add more distilled water to make 250.0 cm‘ of solution. Label this as solution D. Fill a burette with solution C. Using a pipette and a pipette filler, place 25.0 cm’ of solution D into a 250 ml conical flask. Titrate solution D against solution C until the first permanent pink colour is obtained. Record your results in table 2 below. Repeat the titration two more times and complete the table. Retain the remaining solution C for use in question 3.
TABLE II
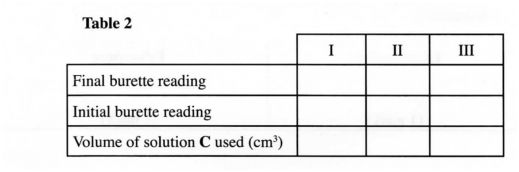
(a) Determine the average volume of solution C used. (1% mark)
(b) Calculate the number of moles of:
(i) aqueous potassium manganate (VII) used; (1 mark)
(ii) iron (II) ions in 25 .0 cm3 of solution D. (1 mole of MnOZ reacts with 5 moles of Fe“). (1 mark
(iii) iron(II) ions in 250 cm‘ of solution D. (1 mark)
(c) Calculate the molar heat of displacement of copper by iron. (2 marks) 2.You are provided with solid E. Carry out the following tests and write your observations and inferences in the spaces provided.
(a) Place all of solid E in a boiling tube. Add about 10 cm’ of distilled water and shake thoroughly. Filter the mixture into another boiling tube. Retain the filtrate for use in test 2
(b) below. Dry the residue using pieces of filter papers.
(i) Transfer about half of the dry residue into a dry test-tube. Heat the residue strongly and test any gas produced using a burning splint.

ii) Place the rest of the residue in a dry test—tube. Add 4 cm’ of 2M hydrochloric acid. Retain the mixture for test (m) below.

iii) To 2 cm-‘ of the solution obtained in (ii) above, add 6 cm-‘ of aqueous ammonia dropwise.

(b) (i) To 2 cm-‘ of the filtrate obtained in (a) above, add about 3 cm3 of aqueous ammonia (Excess).

ii) To 2 cm’ of the filtrate, add about 2 cm’ of 2M hydrochloric acid.

iii) T0 2 cm-‘ of the filtrate, add one or two drops of barium nitrate solution.

3. You are provided with solid G. Carry out the tests in (a) and (b) and write your observations and inferences in the spaces provided. Describe the method used in part (c). (a) Place about one third of solid G on a metallic spatula and bum it in a Bunsen burner flame.
(b) Dissolve all of the remaining solid G in about 10 cm’ of distilled water in a boiling tube. Use the solution for tests
. (i) Place 2 cm-‘ of the solution in a test-tube and add 2 drops of acidified potassium manganate (VII); solution C.

) (ii) To 2 cm‘ of the solution, add all of solid sodium hydrogen carbonate provided.

(c) Determine the pH of the solution obtained in (b) above.

2013 KCSE Chemistry Past Paper-Marking Scheme/Answers
1.a)X is water. or H20
b)It is slightly soluble in water. J and denser than air.
c) Used in hospitals to resuscitate patients. Used in welding when mixed with acetylene in the ocy-acetylene flame. Used by divers and mountaineers. _ Rocket fuel, hospitals for breathing, steel making.
2NaHCO3(S) + com) + H20“) W1)
2AgNO3(s) – > 2Ag(s) + 2NO2(g)+O2(g)
21= 04m -> F6203“) + SO + (1)
3. Crush the seeds in a mortar using a pestle.Add a suitable solvent (acetone / propanone Filter out the solid matter. Evaporate the filtrate to obtain oil.
4. a) Aluminium has a stronger metallic bond because it has more delocalised electrons than sodium.
b) Sulphur has a tinged structure of S8 molecules whiles chlorine is diatomic.
The forces in sulphur are stronger than chlorine.
5. a) It does not sublime.
b) Cut a piece of Sodium metal, place it on a defiagrating spoon, heat it briefly
then lower it into a gas jar of chlorine . It will continue buming forming Sodium Chloride.
6 a) Cu2+(aq) + 2e —> Cu(s)
b) 63.5 g require 2 x 96500 C
l.l84g = 63.5
3598.6 coulombs J (1)
Q = 1.7.92.2.
3586.5 = 2xt 60
35865 =t = 29.988
2
z 30 minutes )
1799.3 s =t (V2)
7 (a) (i) X – Calcium carbide or CaC,
(ii) Y – CH2 = CHCl Chloroethene or vinylchloride
(b) Floor tiles Rain coats Plastic bags
8 Working diagram. a should be deflected
less than B because of its heavier mass.
9. ln water. HCI is ionised ginto H and Cl the Chloride ions are oxidised to chlorine gas by potassium permanganete.
In methylbenzene, HCl remains in molecular gform i.e HCl. The Chloride is not available for oxidation hence no reaction. 10 (a) T (b) l5 g t/111 (c) Fractional crystallization
11 (a) NZHW + Ow ~> NMI + 2H:O
(b) Bond breaking energy
l63 + 4 (388) + 496
= 2211 kJ
Bond making energy
944 + 4 (463)
=_27% kl
Ethalpy change = Bond breaking + Bond making energies.
2211 +(-2796)
= -585 kl/mol
12 a) The acidified permanganete will be decolourised (purple to colourless) b) The permanganate (Vll) is reduced to manganese (II) ion.
(i) A white precipitate forms.
(ii) Ba2+ +SO2-/3 —> BaSO_
13 a) [Zn(NH4/3]2+
b) Zn2= + Mg —> Mg2+ + Zn
14 .Charles Law
At constant pressure. the volume of a fixed mass of gas is directly proportional to its absolute temperature. 1
b) 1 = vs km
P 1v [Ht = I46 dp
_1_r =T l1=l8+._73:36lK
T : P3: 10! km
P1 v =12?
T 1><36l H TI:-
98.39 l-16
T 4849313
14364.94 ~ 1-
r = 273.22 K
15. a) R and T
b) T
16 Zinc granules
The gradient of the graph is less” steep (11 because there is less surface area.
17 NO + O —> 2NO
b) Because nitrogen is inert.
c) Nitrogen (ll) oxide is oxidised to Nitrogen (IV) oxide which is a pollutant. H
18 (a) Water
(b) Bubbles of gas and a white ppt CO2. (P/Q) reacts to give CaCO,
19 (a) These are different forms carbon in the same physical state.
(b) The hexagonal graphite rings have weak Van der Waals forces between the layers that allow the layers to slide over each other (1) while in diamond the atoms are held by strong Covalent bonds. .
20 (a) The atomic radii increase with increase in atomic number. This is due to increase in energy levels.
(b) The group II elements have more protons than group I elements hence this increases the nuclear attraction for the outer electrons. J
21 (a) Cu2 or copper ions
(b) C1 and OH’
22 (a) Copper pyrites chalcocite, malachite
(b) To concentrate the ore
(c) – Brass
– Batteries
23 (a) 100-25=75 cm
(b) CxHy + O2 —> C02 + H20
15 cm’ 75 cml 45 cm
1 5 3
Cx Hy =-Iy + 5 O2—> 3 CO2 + 4 H20
x = 3 y = 8
C3H8 t/(1)
24 Ca(NO3)2 —> Ca + 2NO3
RMM of Ca(NO)2 = 164
Concentration of Ca(NO3)2 = 4.1
Conc. in g/l
RMM
Molarity =
= Q
164
= 0.025M J M)
1 mole Ca(NO3)2 E 2 moles Nitrate
0.025 iii E 2 0.025
0.05M
25 It would remain unchanged (1)
There is no water to form hypochlorous acid
26 When aqueous sodium chloride is added to Ca2 . There is no ppt while a white ppt is formed when aqueous sodium chloride is added to a solution containing Pb
27 (a) N. being a weak acid provides few H’ to be neutralised by OH” hence there is a slight increase in temperature.
(b) CH3COOH + KOH ) —> Cl-l3COOKmq] + H20“)
28 (a) Experiments 1 and 3. J
(b) In experiment 1, the ions in K2CO3 are tightly held in position and cannoot move V
while sugar solution does not have ions that can carry a current in solution.
29 1 H mass 18
2
1 H mass 20

