2013 KCSE General Science Past Paper
4.6.1 General Science Paper 1 (237/1)
SECTION A: BIOLOGY (34 marks)
Answer all the questions in this Section in the spaces provided.
1. (a) What is meant by the term botany? (1 mark)
(b) State two rules of binomial nomenclature. (2 marks)
2. (a) Give one function for each of the following parts of a light microscope: (2 marks)
(i) mirror;
(ii) rotating nose.
(b) Distinguish between a tissue and an organ system. (2 marks)
3. (a) What is meant by active transport? (1 mark)
(b) Give one role of each of the following in plant roots: (2 marks)
(i) active transport;
(ii) osmosis.
4. (a) Name the region of the alimentary canal where amino acids are absorbed. (1 mark)
(b) Other than provision of food, state another importance of photosynthesis to animals. (1 mark)
5. (a) State two features of the leaf epidermis that allows light to pass through. (2 marks)
(b) What are the functions of the following minerals in the human body? (2 marks)
(i) Iron.
(ii) Calcium.
6. (a) State two environmental conditions that contribute to low rate of transpiration in plants. (2 marks)
(b) Give one function of each of the following components of blood: (2 marks)
(i) white blood cells;
(ii) platelets.
7. (a) Name the causative agent of whooping cough. (1 mark)
(b) State what happens to the following structures on the chest cavity during inhalation:(2 marks)
(i) diaphragm:
(ii) rib cage.
8. (a)Name two products of anaerobic respiration in plants. (2 marks)
(b) Give two adaptations of blood capillaries to their function. (2 marks)
9. The diagram below represents part of a human organ.
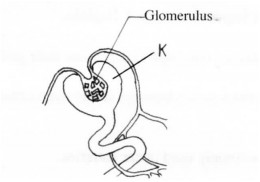
a)Name the structure labelled K. (1 mark)
b)Explain why contents of K include non excretory substances in a healthy person.(2 marks)
10. Describe how diabetes mellitus occurs. (2 marks)
Explain the importance of sweating in regulating human body temperature. (2 marks)
SECTION B: CHEMISTRY (33 Marks)
Answer all the questions in this Section in the spaces provided.
11 A mixture contains ammonium chloride, sodium chloride and sand. Describe how one can separate and recover the substances in the mixture. (3 marks)
12 The curves below were obtained by a student after heating two solid substances.
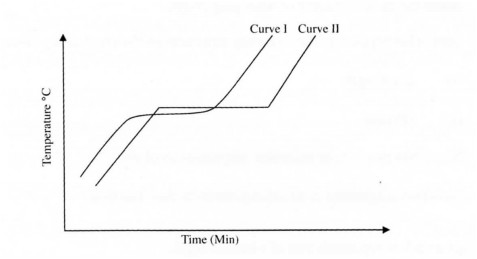
(a) Which curve represents an impure substance? Explain. (11 marks) 13 a)Write a word equation for the reaction between dilute hydrochloric acid and calcium hydrogen carbonate. (1 mark)
(b) Name the acid which is commonly used in car batteries. (1 mark)
14 The diagram below shows how gas Q is prepared in the laboratory.
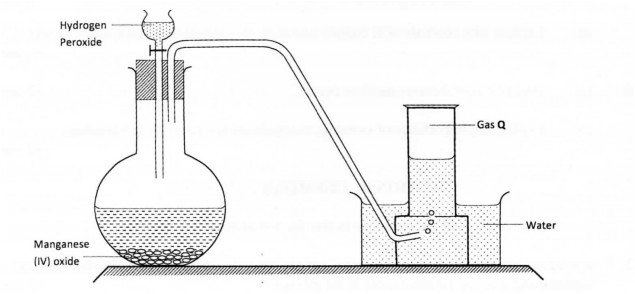
(a) Identify gas Q. (1 mark)
(b) If manganese (IV) oxide was removed, what would be the effect on the reaction progress? Explain. (2 marks)
(c) State one property of gas Q that enables it to be collected as shown in the diagram. (1 mark)
15 The diagram below illustrates an experiment where dry hydrogen gas is passed over heated Magnesium Oxide
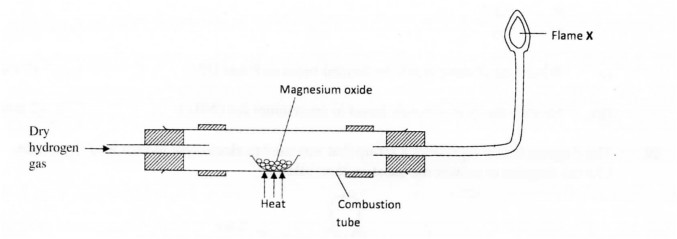
(a) State the observation that is made in the combustion tube. (1 mark)
(b) Explain the observation made in (a) above. (1 mark)
(c) What substance burns at flame X? (1 mark)
16 (a) Study the table below and fill in the blank spaces. The letters do not represent the actual symbols of the elements.
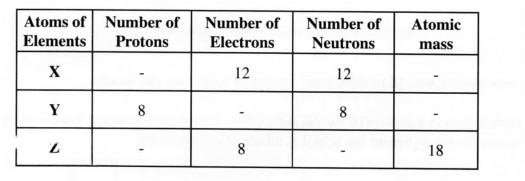
(b) Which atoms are isotopes of an element? (1 mark)
17 Acids and bases are categorized as either strong or weak.
(a) What is meant by the term weak acid? (1 mark)
(b) Give one example of each of the following:
(i) strong alkali; (1 mark)
(ii) strong acid. (1 mark)
18 The electronic configurations of P and U (not actual symbols of elements) are shown below. Use the information to answer the questions that follow.
P 2.8.2
U 2.6
(a) What type of bond would be formed between P and U? (1 mark)
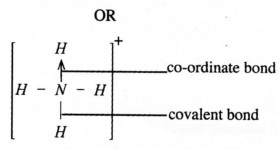
(b) Identify the type of bonds found in ammonium ion (NHI ). (2 marks)
19 The diagram below represents a set-up that was used to electrolyse molten lead (II) iodide. Use the diagram to answer the question that follows.
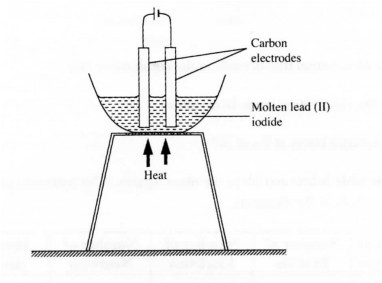
Why was molten lead (II) iodide used instead of solid lead (II) iodide. (2 marks)
20. The table below is a section of the periodic table. Use it to answer the questions that follow. The letters do not represent the actual symbols of the elements.
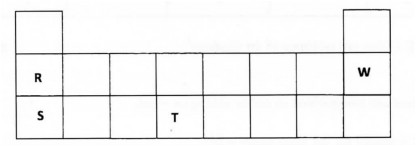
(a) How do the ionisation energies of R and S compare? Explain. (2 marks)
(b) Write the electronic configuration of W. (1 mark)
(c) To which group and period does element T belong?
(1) Group……………………………….(1 mark)
(ii) Period …………………………….(1 mark)
21 (a) A student put lead (II) carbonate and lead (II) nitrate in separate test tubes and performed the tests as shown in the table below. Complete the table by giving the expected observations.
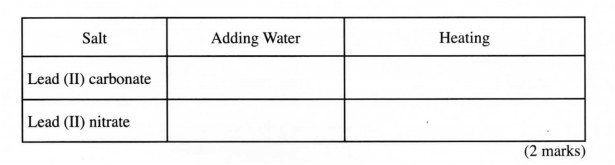
Salt Adding Water Heating
Lead (II) carbonate
Lead (II) nitrate
(2 marks)
(b) State one use of calcium hydroxide. (1 mark)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this Section in the spaces provided.
22 Figure 1 shows a burette containing some liquid after 8 g of the liquid was drained out. If the level of the liquid was initially at the 10 cm3 mark, determine the density of the liquid.
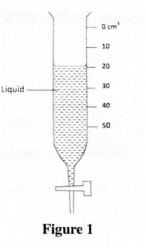
Figure 1
23 When a drop of water is placed on a clean metal surface, it wets the surface. Explain this observation in terms of the forces involved. (3 marks)
24 Figure 2 shows a simple mercury barometer set up in a physics laboratory.
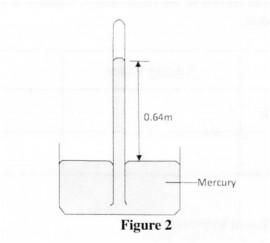
The height of the mercury column is 0.64 m. Given that the density of mercury is l3600 kgm and acceleration due to gravity, g is 10 ms“, determine the atmospheric pressure in Nm2. (3 marks)
25 A student in a room observed a beam of sunlight entering into the room from a hole on the roof. The student noted that dust particles illuminated by the beam were moving in random motion. Explain how this motion was caused. (2 marks)
26 Figure 3 shows a glass container being used to heat a liquid. The wire gauze is placed between the container and the flame.
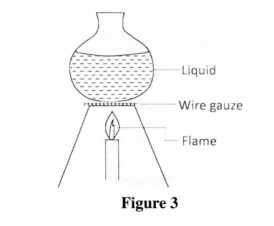
Explain how the wire gauze prevents the glass container from cracking. (3 marks)
27 State two properties of mercury that make it a suitable liquid for use in thermometers. (2 marks)
28 Figure 4 shows a uniform plank of length 4 m and of weight 50 N. It is pivoted at a distance x from one end and balanced horizontally by a weight of 30 N.
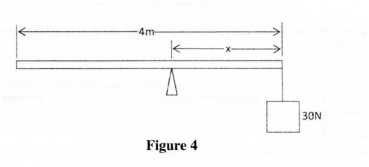
Determine the value of x. (3 marks)
29 Figure 5 shows a block of wood with a hollow part. The block is resting on a horizontal bench.
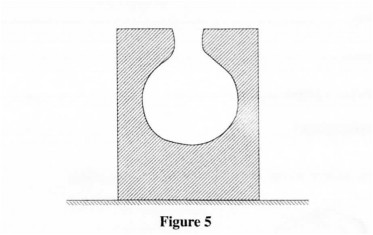
Explain the effect on the stability of the block when sand is used to fill the hollow section. (2 marks)
30 An object of weight 25 N extends a spring by 0.4 cm. Determine the weight of an object that would extend the spring by 0.96 cm. (3 marks)
31 A car starts from rest and accelerates uniformly for 4 seconds. It attains a velocity of 15 ms2 and maintains it for 3 seconds. Sketch a velocity time graph for the motion of the car within the 7 seconds. (3 marks)
32 A body is pulled along a horizontal surface at a constant velocity. State two factors that affect friction between the body and the surface. (2 marks)
33 State the energy changes that take place as a building block drops from the top of a building to the ground. (2 marks)
34 Two copper spheres A and B of the same size are placed in a container. Sphere A is hollow while B is solid. When the container is filled with water, it is observed that A floats while B sinks. Explain this observation. (2 marks)
4.6.2 General Science Paper 2 (237/2)
SECTION A: BIOLOGY (34 marks)
Answer all all questions in this section on the spaces provided
1. In the table below. name the causative agent and start

2. (a) State the functions of each of the following structures in human beings:
(i) ovary;
(ii) uterus;
…………………………. ..
(iii) Cowper’s gland…………………………………………….. ..
(b) What is implantation?
3. The diagram below represents an external view of a maize grain.

Name the parts labelled A, B and C.
A ………………………………………………………. ..
C ……………………………………………………….. ..
4. (a) What is fertilization?
(b) With an example, describe discontinuous growth.
5 (a) What is variation as used in biology?
(b) Explain the following terms:
(i) haploidy;
(ii) genotype;
(m) dominance.
6 State two applications of Genetics other than Genetic Counselling and Engineering.
7 (a) Explain the following terms:
(i) niche; …………….. ..
(ii) carrying capacity.
…………. ..
(b) Describe the origin of life by special creation.
8 Describe the structure of a sensory neurone.
9 (a) State one survival value of geotropism to plants.
(b) State two functions of auxins.
10 State the importance of support and movement in plants.
(i) …………………………………. ..
(ii) ………………………………… ..
(iii)……………………………….. ..?
SECTION B: CHEMISTRY (33 marks)
Answer all the questions in this section in the spaces provided.
11 (a) Name the compound whose structure is given below.
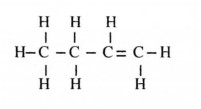
(b) Name two reagents that can be used to distinguish between alkanes and alkenes. (1 mark)
(c) State two uses of alkenes. (2 marks)
12. (a) Name two common ores of iron. (1 mark)
(b) Describe the reduction process in extraction of iron metal from its chief ore. (3 marks)
(c) State any one use of wrought iron. (1 mark)
13. The flow chart below shows part of the process for manufacturing sulphuric (VI) acid.
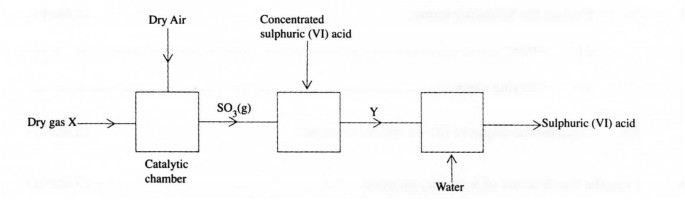
(i) X;…………………………………………..
(ii) Y …………………………………………..
(l mark)
(b) Name the catalyst used in the catalytic chamber. (l mark)
(c) Explain why sulphur (VI) oxide is first dissolved in concentrated sulphuric (VI) acid and not in water. (2 marks)
14. The graph below shows the rate of production of hydrogen gas when zinc granules are reacted with excess 2 M hydrochloric acid. The hydrogen gas produced was collected in a syringe.
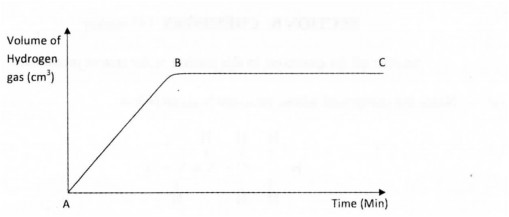
(a) Explain why part BC of the graph is horizontal. (2 marks)
(b) On the same axes, sketch the curve expected if zinc powder of the same amount as the zinc granules was used. (1 mark)
(c) What will be the effect of using excess l M hydrochloric acid instead of excess 2 M hydrochloric acid. (1 mark)
15. The set-up below was used to prepare chlorine gas. Use it to answer the questions that follow.
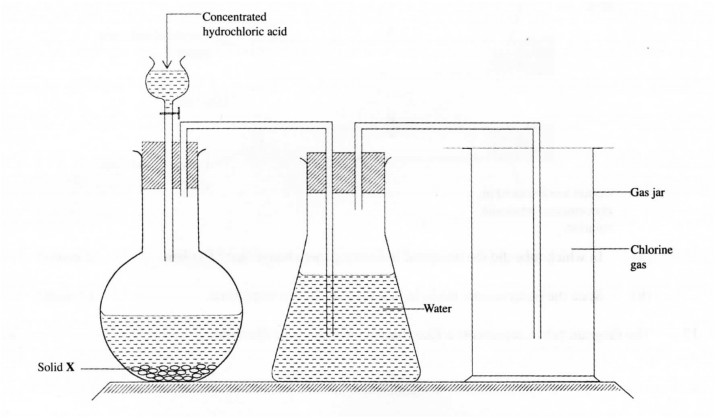
(a) Name solid X.
(1 mark)
(b) Why was the chlorine gas produced in the set -up above passed through water before collection. (1 mark)
(c) State two observations made when a moiste d b ne lue litmus paper was introduced into the gas J81’ containing chlorine gas. (1 mark)
ed to investigate the rates of difiusion of ammonia and hydrogen
16 The set-up below was us solutions of hydrochloric chloride gases. Pieces of cotton wool were soaked in concentrate acid and ammonia respectively, and inserted into the glass tubes A and B of the same size at the same time. (H = 1.0; Cl = 35.5; N = 14.0).
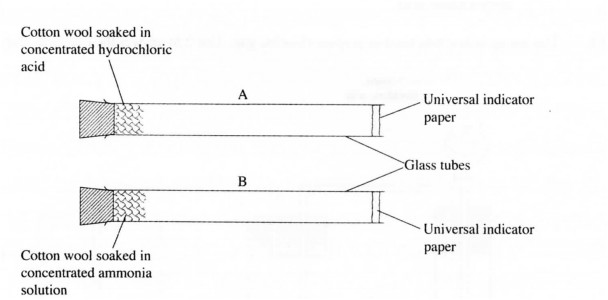
(a) In which tube did the universal indicator paper change first? Explain (2 marks)
(b) State the observations made in tubes A and B after some time. (1 mark)
17 The diagram below represents a Jiko (burner) with burning charcoal.

(a) (i) State two products formed in regions M and N. (1 mark)
M ………………………………. ..
N ……………………………….. ..
(ii) What is the function of the part labelled L. (1 mark)
(b) Why should people be discouraged from using charcoal in Kenya? (1 mark)
(c) Give two advantages of using kerosene over charcoal as a fuel. (2 marks)
18. Given that 25 g of compound XCO3 contains 0.25 moles, calculate the relative atomic mass of X. (C = 12.0; O = 16.0). (2 marks)
Describe how one can prepare one litre of 0.5 M magnesium nitrate solution. (Mg = 24.0; N = 14.0; O = 16.0). (3 marks)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this section in the spaces provided.
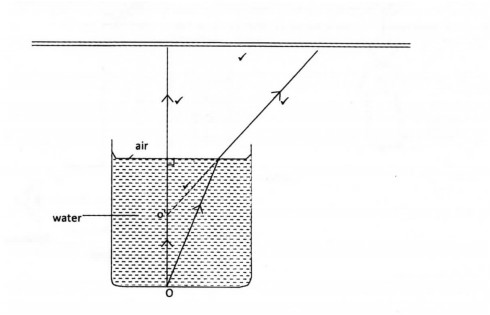
20. Figure 1 shows two rays of light from a point O at the bottom of a beaker full of water. The rays are refracted into the air at the surface of the water.
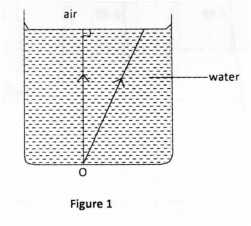
On the diagram, complete the path of the rays in air to show the position of the image O of the point. (3 marks)
21. State the reason why it is not possible to charge a metal rod while holding it with a bare hand. (1 mark)
22. Explain why a naked flame should be avoided in an enclosed place, where an acid accumulator is being charged. (2 marks)
23. A student is given a magnet with its ends marked N and S. She is also given a metal bar with its ends marked A and B. Explain how the student can prove that the metal bar is a magnet.
24. (a) State what is meant by transverse waves. (1 mark)
(b) Figure 2 shows how the displacement of a point varies with the time as a wave passes
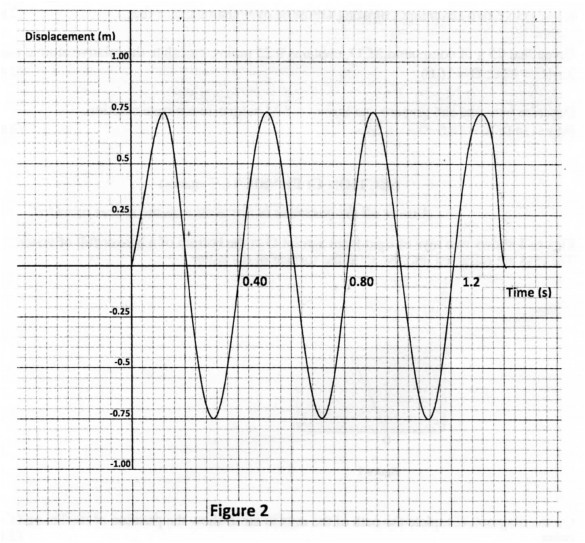
(i) the amplitude. (1 mark)
(ii) the frequency of the wave. (1 mark)
25 State two factors that affect the speed of sound in air. (2 marks)
26 (a) State the reason why, when measuring current through a resistor, the ammeter is always connected in series and not in parallel with the resistor. (1 mark)
(b) Figure 3 shows the scale of a voltmeter in a circuit. The scale of the voltmeter is in volts.
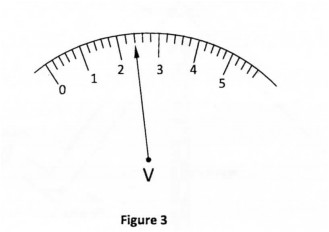
State the reading shown. (1 mark)
27 Figure 4, shows a circuit consisting of two different coils A and B connected in series with a battery and a switch. The coils are immersed in equal amounts of water in beakers P and Q.
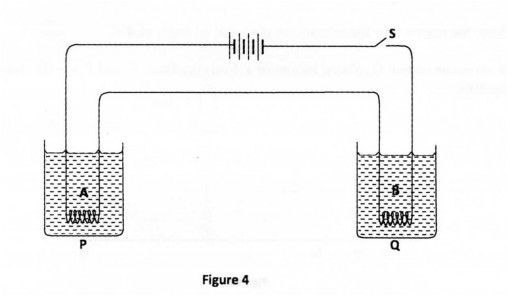
After switching on the circuit it is observed that water in Q boils before the water in P. State a reason for the observation. (1 mark)
28 Figure 5 shows a periscope being used to observe a player over a wall.
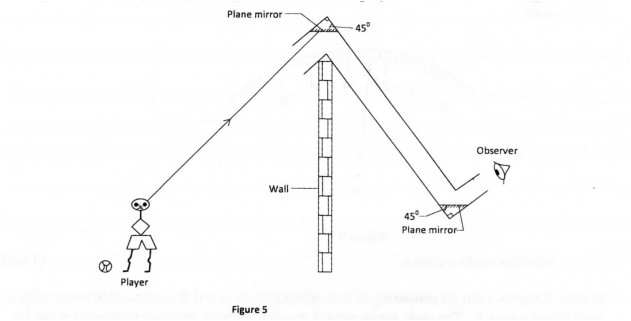
(a) Complete the diagram to show the path of the ray from the player to the observer. (l mark)
(b) State the reason why the mirrors are placed at an angle of 45°. (l mark)
29 Figure 6 shows an object, O, placed infront of a diverging lens. F I and F2 are the two principal foci of the lens.
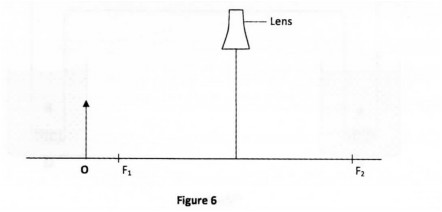
On the figure, draw a ray diagram to locate the image formed. (3 marks)
30 State two differences between hard X-rays and soft X-rays. (2 marks)
31 State two functions of the anode in the electron gun of a Cathode Ray Oscilloscope. (2 marks)
32 An electric bulb marked 75 W is used for 4 hours in a day. Determine the quantity of energy used in seven days in Kilowatt-hours. (3 marks)
33 Explain how pure silicon can be changed into a P-type semi-conductor by doping. (3 marks)
34 The mass of a 50 g radioactive material is found to have reduced to 6.25 g in 30 hours. Determine the half life of the material. (3 marks)
2013 KCSE General Science Past Paper-Marking Scheme/Answers
5.6.1 General Science Paper 1 (237/1)
SECTION A: BIOLOGY
1. a) The study of plants; (1 mark)
b) It gives two names to an organism, generic and specific names; The generic name starts with a capital letter while the specific name starts with a small letter; the names should be underlined / or italicized; – (2 marks)
2.a)(i) Directs/reflect light onto the specimen;
(ii) Places desired objective lens into position;
(2 marks)
b) Tissue – a group of similar cells performing a function;
Organ system – a group of (connected) organs functioning as a unit; (2 marks)
3. a)Movement of substances against concentration gradient across cell membranes using energy; (1 mark)
b) (i) Absorption of ions/mineral salts; (1 mark)
(ii) Absorption of water; (1 mark)
4. Small intestines/ileum; (1 mark)
b) Provision of oxygen; (1 mark)
5. a) Transparent / have no chlorophyll;
Thin/one cell thick; (2 marks)
b) (i) Formation of blood; (1 mark)
(ii) Fonnation of teeth and bones;
Participates in blood clotting. (1 mark)
6 a) Low temperature; high humidity/high soil water;
Low wind velocity; low light intensity;
First two correct. (2 marks)
b) (i) Defence; (1 mark)
(ii) Participates in blood clotting; (1 mark)
7. (a) Bardetella pertussis 0728 ho Q3
(b) (i) Diaphragm flattens;
(ii) Rib cage is lifted upwards and outwards.
8. (a) Carbon dioxide; alcohol; energy;
First two correct.
(b) Thin walled to reduce diffusion distance;
Numerous to increase surface area;
Moist to dissolve diffusing substances;
First two correct.
9. (a) K – Bowman’s capsule;
(b) Ultrafiltration; forces all small molecules into the Bowman’s capsule; before useful ones can be re-absorbed back again.
10. (a) Failure of the pancreas to secret enough insulin
Failure of the liver to convert glucose into glycogen;
leading to excess sugar in the blood;
(b) When it is hot, sweat is produced on the skin;
The sweat uses heat from the body to evaporate thereby cooling the body; (Latent heat of vaporisation)
SECTION B : CHEMISTRY (33 marks)
11. Heat the mixture √ for ammonium chloride to sublime √ and collect the sublimate; √ Add water to dissolve sodium chloride and decant / filter √ (W to obtain sand as the residue and sodium chloride solution; √ Evaporate sodium chloride solution to dryness √ to obtain sodium chloride crystals.
OR
Add water, filter off sand, carry out fractional crystallization, to obtain NaCl(s) filter off NaCl(s) evaporate filtrate to dryness to obtain NH ‘Cl.
12.(a) Curve I (V2) ; (3 marks)
Curve I does not have definite temperature change / constant temperature change. (1)
(b) Melting point.
13. (a) Calcium hydrogen carbonate + Dilute hydrochloric acid —> Calcium chloride + Carbon (IV) oxide + Water;
(b) Sulphuric (VI) acid. or sulphuric acid
14.a) Oxygen / O2
b) Reaction slows down / less production of gas Q
Manganese (IV) oxide is a catalyst or increases rate of decomposition of hydrogen peroxide. (2 marks)
c ) Gas Q slightly soluble in water. (1 mark)
15 a)White magnesium oxide remains white. √ (1 mark)
b) Hydrogen is below magnesium in the reactivity series hence it can not reduce its oxide.
OR
Hydrogen is less reactive than magnesium, so it cannot reduce magnesium oxide. (1 mark)
c) Hydrogen gas/H2. (1 mark)
16. (a)
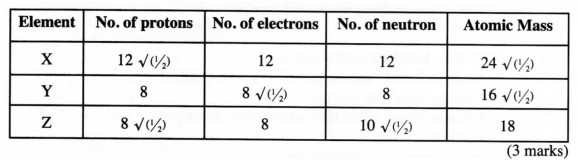
b) Y and Z are isotopes (1 mark) 17. a) Weak acid is one that does not ionize/dissociate completely in aqueous solution. √ (1) (1 mark) b) (i) Sodium hydroxide or potassium hydroxide. (1 mark) (ii) Sulphuric (VI) acid or Hydrochloric acid / Nitric (V) acid/Nitric acid or Sulphuric acid. (Accept correct formulae) (1 mark) 18 a) Ionic bond/ Electrovalent . (1 mark)
Covalent bonds √ (1)
b)Co-ordinate/ Dative bond (2 marks)
19. In the molten lead (H) iodide, the ions are mobile hence conducts electricity while in solid lead (H) iodide, the ions are at fixed positions hence does not conduct electricity. (2 marks)
20. (a) Ionisation energy for R is higher than that of S . R is smaller in size than S making outer electron in R more difficult to remove since nuclear attraction on outermost electrons in R is higher (Lg. (2 marks)
(b) 2.8 x/(I) (1 mark)
(C) (i) Group 4 (3 mark)
(ii) Period 3 vw (1 mark)
21. (a)
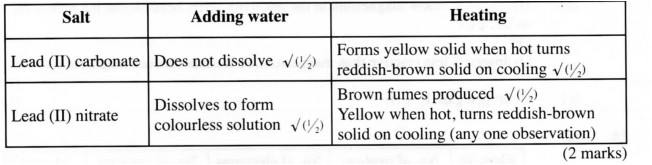
(b) – Making builder’s mortar and plaster
– In agriculture to reduce/prevent too much acidity
– Making bleaching powder
– For detecting Carbon (IV) oxide gas in laboratory
– In softening hard water
– In scrubbing in contact process
(Any 1 correct)
(1 mark)
SECTION C : PHYSICS
22. Volume = 20 – 10
= 10 cm‘
Density = Mass
Volume
= 8
10
= 0.8 gem”
23. The forces involved are cohesive and adhesive forces
The adhesive forces between the water molecules and the metal surface is greater √ than the cohesive forces between water molecules.
24. Pressure = h p g
= 640 >< 1.36 >< 10‘ X 10
1000
= 87040 Nm √
25. The large dust particles are being bombarded by the tiny air particles which are in continuous random motion.
26. (a) The wire gauze prevents the glass from being heated at one point, (b) Since the wire gauze is a good conductor √ it conducts the heat evenly to a large area of the glass container.
27. – It is a good conductor of heat.
– lt is visible (opaque).
√ – It has a wide range of temperature (high boiling point and low freezing point).
– It expands / contracts unifonnly.
√ (any two correct)
28. Clockwise moment = Anticlockwise moment
√ 30 >< x = 50(2 – x)
30x = 100 – 50x
80x = 100
5 = l .25m
29. The Center of gravity is raised thus reducing the stability √ of the block.
30. F = Kx K = 25
√ 0.4
F = 25 >< 0.96
√ 0.4
= 6ON √
30. – labelled axis √
– accelerating for first 4 seconds √
– uniform velocity between 4 seconds
and 7 seconds √
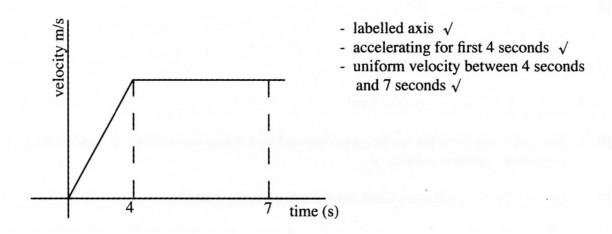
I I
4 7 time (S)
31. – The reaction force from the supporting surface. √
– Nature of the surfaces in contact. J
Potential √ √ kinetic √ i> sound/heat
(a) The sphere that floated was hollow while the other one was a solid sphere.
(b) The floating sphere experienced an upthrust equal to its own weight. √
The sinking sphere experienced an upthrust lower than its own weight. √
5.6.2 General Science Paper 2 (237/2)
SECTIONA: BIOLOGY
1. (a)

2. (a) (i) Ovary – produces eggs / ova ; and female hormones;
First one correct. (1 mark)
(ii) Uterus – where the embryo develops;
Contraction of the walls aids in the expulsion of the developed foetus during birth I parturition;
First one correct. (1 mark)
(iii) Cowper’s gland – secrets an alkaline fluid that neutralizes the acidity along the urethra; (1 mark)
(b) Attachment of the blastocyst to the walls of the uterus; by the villi. (1 mark)
3. A – Pericarp fused with testa;
B – Position of plumule;
C – Position of radicle;
(3 marks)
4. (a) The fusion of nucleus of male gamete / sperm with the nucleus of female gamete / ovum; to form a zygote; (2 marks)
(b) In a discontinuous growth, the organism shows a number of periods of rapid growth followed by long periods when no growth occurs; e.g. Growth shown by arthropods; (an example of an arthropod like locust, crab etc). (2 marks)
5. (a) Variation – the differences in traits that occur among members of the same species; (1 mark)
(b) (i) Haploidy – Chromosome numbers that are half of the full complement; (1 mark)
(ii) Genotype – refers to the genes that an organism contain / have for a particular trait. Genetic composition of an organism. (1 mark)
(iii) Dominance – refers to the genes that determine the expression of the genetic trait in offspring; State where genes express/supress other genes. (1 mark)
Blood transfusion; plant / animal breeding; crime detection, disputed parentage (2 marks) 6. (a) (i) Niche – the position that an organism occupies in a habitat / a functional description of a species role in a community / an expression of the range of all the factors that influence whether a species has all the resources it needs and whether it can carry out all the activities necessary for survival and reproducing; (1 mark)
(ii) Carrying capacity – the maximum population / number of organisms of a particular speies that can be sustained by a given supply of resources; in an environment. (1 mark)
(b) Special creation – life was brought into existence / created by a supreme being / God; life was created in perfect forms and have remained unchanged over time; (2 marks)
Sensory neurone – it has a cell body; situated off the axon.
Has receptor dendrites; located in the sensory organ.
Has long dendron and short axon;
Has myelin sheath; with nodes.
First three correct. (3 marks)
7.(a) Geotropism – roots move towards source of water; Plants get anchored in the soil;
First one correct.
(1 mark)
(b) Auxins – promote / initiates growth; adventitious root development; causes apical dominance;
Prevent ageing / senescence;
Responsible for tropic movements;
First two correct. (2 marks)
10.Importance of support and movement in plants.
– At cellular level, like growth of pollen tube to bring about fertilization;
– At organ level such as tropic movements for survival value;
– Enable plants to get resources from the environment such as light / water nutrients;
– For escape to avoid harmful stimuli such as temperature;
– Bearing of leaves, fruits
First three correct. (3 marks)
SECTION B : CHEMISTRY (33 marks)
11.a) But-l-ene. /butene
b) Bom1ine water.
Acidified potassium manganate (VII). /KMnOJ V
c) Ripening of fruits.
Manufacture of plastics.
Manufacture of detergents
Manufacture of ethan-I . 2-diol
Manufacture of ethanol through hydrolysis
(Any 2 correct.) (2 marks)
12. a)haematite up magnetite :1 (1 mark)
b) Coke in the fumace burns in the hot air to form carbon (IV) oxide Carbon (IV) oxide J (1) rises to the middle of the fumance and reacts with more coke to fonn carbon (II) oxide Carbon (II) oxidel coke reduces the Iron (Ill) oxide to the Iron metal and carbon (IV) oxide. (3 marks)
c) Making Agricultural implements, nails, sheets, omaments and horse-shoes. (1 mark)
(Any 1 correct.)
13. a) X – Dry Sulphur (IV) oxide / dry SO2 sulphur dioxide
b) Y – Oleum (V2) / I-IZSZO7 (1 mark)
c) Vanadium (V) oxide / Vanadium Pentoxide (1) (l mark)
or Platinum/platinised asbestos.
Dissolvinp SO; in water is an exothermic reaction that makes the acid to vaporise (11 (2 marks)
14.a) The reaction is over since all the zinc granules have been used up. (2 marks) b)On the graph
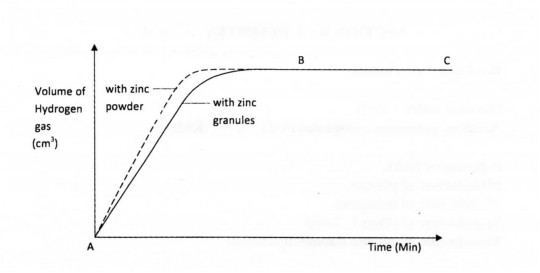
(c) The rate of reaction will be slower. (1 mark)
15) a)Potassium manganate (VII)/CaOCl2 a (1 mark)
b)To remove the more soluble fumes of hydrogen chloride gas produced by the acid. (1 mark)
c)The moist blue litmus paper tums red.
The red litmus paper is then bleached. (V2) (1 mark)
16 a) B /NH 3
Ammonia gas (RMM 17) is less dense Vzlthan hydrogen chloride gas/hydrochloric acid gas (RMM = 36.5) and hence diffused faster. (2 marks)
b) In glass tube A, the universal indicator tumed Red, while in glass tube B, the universal indicator tumed green. (1 mark)
17 (i) MI Carbon (IV) oxide (COz) , N: Carbon (II) oxide (CO) . (1 mark)
(ii) To allow in air. 1/(1) (1 mark)
It brings about defforestration.
global warming / Green house effect
(1 mark)
(Any 1 correct.)
iii – Easier to store it is less bulky
– Amount of energy produced per unit
amount is higher in kerosene than charcoal. i.e. Kerosene has high heating value than charcoal.
– It is a cleaner fuel compared to charcoal.
(any 2 correct) (2 marks)
18. mass (g)
RPM: N0. of moles
RFM= (V1)
= 100 M)
x+6O= 100 (V2)
x = 40 1/(V2)
(2 marks)
19. RFM of Mg(NO3)2 = 148
0.5 mole of Mg (N03): = 0.5 x 148
= 74 g 1/(V1)
b) .Weigh 74 g of magnesium nitrate and place it in 500 cm’ beaker. (V1) Add about 400 cm’ of distilled water and stir to dissolve Mg (NO3)z. ) Transfer solution to a litre volumetric flask . Rinse beaker and pour the solution into the volumetric flask. Top up the remaining volume with distilled water upto the mark. (V2) (3 marks)
SECTION C : PHYSICS
20.
21. Any acquired charge flows through the body.
22. During charging process both Oxygen and hydrogen gas are given oft‘. The two can become explosive ifexposed to u naked flame.
23. The bar is 21 magnet if any of it ends is repelled by the magnet North or South poles.
24. (a) Waves in which the vibration of the particles is always perpendicular to the direction of the wave travel.
(b) (i) 0.75m
25. – Density
– Pressure
– Humidity/temperature
= 2.5 Hz.
26. (a) All the current passing through resistor passes through the ammeter.
(b) 2t4V 27. Coil B has higher resistance than A.
28. (a) (i)div class=”ImageBlock ImageBlockCenter”>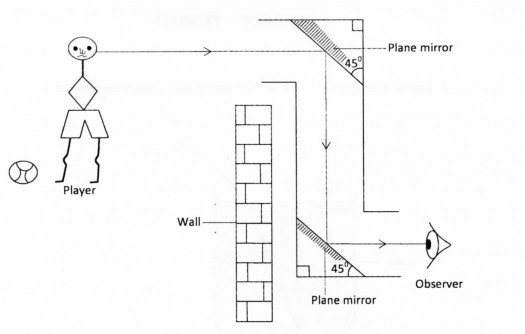 (b) The ray successively passes through the tube (Ray is parallel to the walls of the tube.
(b) The ray successively passes through the tube (Ray is parallel to the walls of the tube.
29.
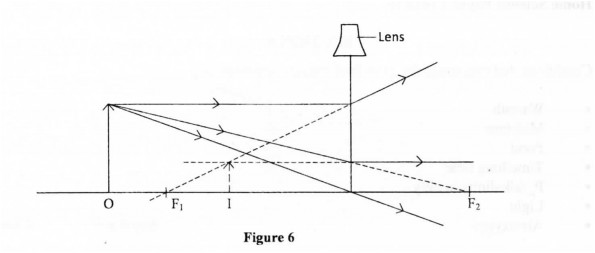
30. Hand x-rays have higher penetration power than soft x-ray.
Hard x-rays are produced at higher accelerating voltage than soft x-ray.
Hard x-rays have shorter wave length than soft x-rays.
31. – Accelerating the electrons.
– Focusing the electrons into a fine beam.
32. E = Pt
A
1000 X 4 X 7
= 2.1 Kilowatt – hours p> (any correct two)
33. Pure silicon is doped with a trivalent element. This results in the three valency electrons of the impurity pairing with electrons of silicon and thus leaving a hole in the structure.
34. 50g —> 25g —> l2.5g —> 6.5g
Three half lifes = 30 hrs
Half-life = 10 hrs

