2014 KCSE General Science Past Paper
3.7.1 General Science Paper 1 (237/1)
SECTION A: BIOLOGY (34 marks)
Answer all the questions in this section in the spaces provided.
1 (a) State one precaution that should be observed to protect bench tops when performing laboratory experiments.(1 mark)
(b) State the importance of transport in plants.(3 marks)
(i) ………………………………….
(ii)………………………………….
(iii)…………………………………
2 The diagram below represents a terrestrial plant.
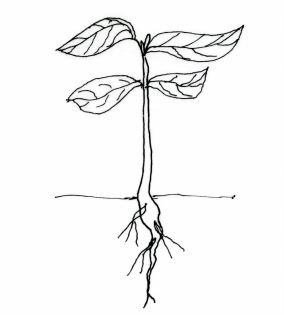
Based on the observation of the diagram:
(a) state the class to which the plant belongs;(1 mark)
(b) give a reason for your answer in 2(a) above.(1 mark)
3 A strip of raw pawpaw measuring 3 cm in length was immersed in a beaker of water. After 30 minutes the strip measured 3.2 cm.
(a) Explain the change in length.(2 marks)
(b) State one other observation that was made on the raw pawpaw strip at the end of the experiment.(1 mark)
4 The diagram below represents a plant cell as seen under high power of the light microscope.
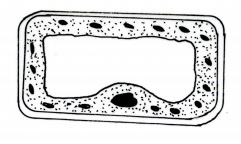
(a) Based on observations of the diagram, give three reasons why it is a plant cell.(3 marks)
(b) Name the organelle that carries out autotrophic nutrition in plants.(1 mark)
5 Give a reason in each case why enzymes are:
(i) catalysts;(1 mark)
(ii) denatured by high temperatures.(1 mark)
(b) Explain the effect of temperature on the rate of photosynthesis.(2 marks)
6 (a) State two reasons for carrying out vaccination.(2 marks)
(b) What is an allergic reaction?(1 marks)
7 (a) Explain how age affects the rate of breathing in human beings.(2 marks)
(b) Name the causative agent of pulmonary tuberculosis.(1 mark)
8 (a) The diagrams below represents an experimental set up that was used to investigate the process of respiration.
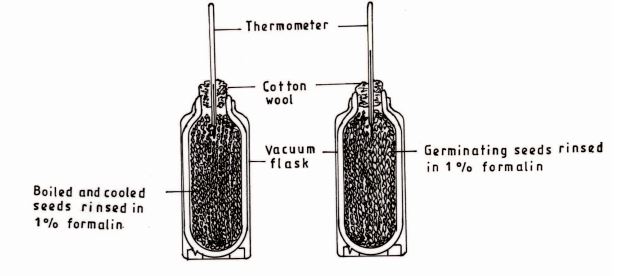
(i) State the aim of the investigation.(1 mark)
(ii) State the purpose of rinsing the seeds in 1% formalin. (1 mark)
(b) Name two end products of digestion of a meal consisting of boiled rice and beans without oil.(1 mark)
9 (a) Apart from thermoregulation, state two other roles of the skin in homeostasis.(2 marks)
(b) How does amoeba maintain osmotic pressure when placed in hypotonic solution?(2 marks)
10 How is the liver involved in thermoregulation?(3 marks)
SECTION B: CHEMISTRY (33 Marks)
Answer all the questions in this section in the spaces provided.
11 An experiment was set up to investigate the products of a burning candle as shown in the diagram below.
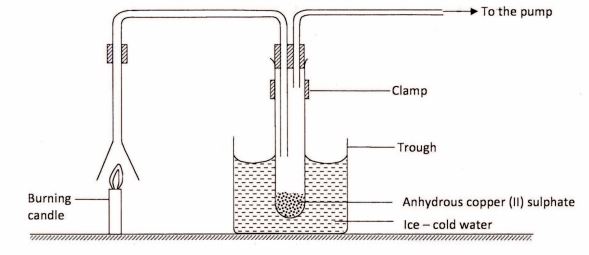
(a) (i) State an observation made on the anhydrous copper (II) sulphate. (1 mark)
(ii) Explain the observation made in a(i) above. (1 mark)
(b) What effect does the other product of burning candle have on the environment? (1 mark)
12 The diagram below is a set-up for the laboratory preparation of oxygen gas.
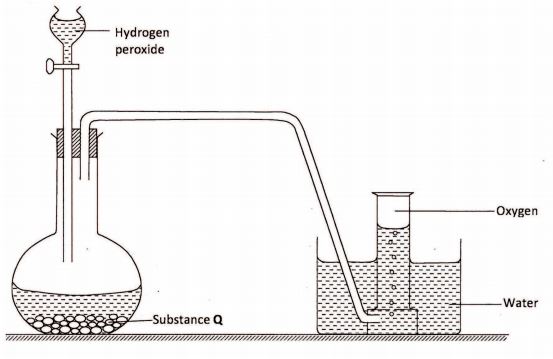
(a) Name substance Q.(1 mark)
(b) Write an equation for the reaction that produces oxygen gas.(1 mark)
(c) Name two other reagents which can be used to prepare oxygen in the laboratory.(1 mark)
(d) Describe a laboratory test to show that the gas produced is oxygen.(1 mark)
13 The table below shows the pH values of solutions.
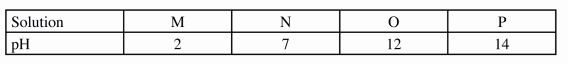
(a) Which solution is likely to be calcium hydroxide?(1 mark)
(b) Name the products formed when solution M reacts with sodium carbonate.(1 mark)
14 (a) (i) What is meant by the term “hygroscopic salt”?(1 mark)
(ii) Give one example of an hygroscopic salt.(1 mark)
(b) State one use of sodium chloride other than being a food additive.(1 mark)
15 A student set up an experiment to investigate the effect of passing an electric current through lead (ll) bromide using graphite electrodes as shown below.
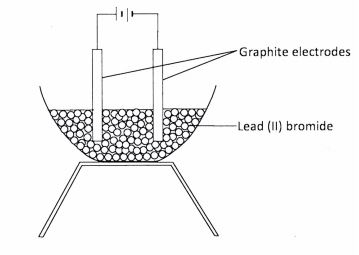
(a) Identify a mistake in the set-up. (1 mark)
(b) Name the product formed at the anode after the mistake was corrected. (1 mark)
16 (a) Hard water is healthy for drinking. Explain. (1 mark)
(b) Explain how ion-exchange is used to remove hardness in water. (2 marks)
17 A student used paper chromatography to separate pigments in different inks and obtained the results shown in the diagram below.
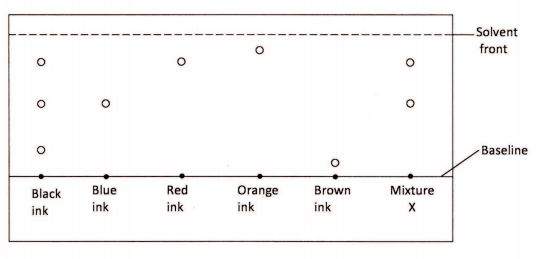
(a) Identify the inks that are in X. (1 mark)
(b) Which of the inks is least soluble? (1 mark)
18 Describe how sodium chloride can be obtained from a mixture of sulphur powder and sodium chloride. (3 marks)
19 (a) What is meant by the term “ionization energy”? (1 mark)
(b) Study the following table and use it to answer the questions that follow. The letters do not represent the actual symbols of the elements.
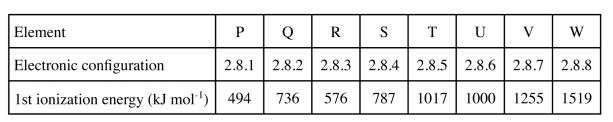
(i) From the table, ionization energies increase generally from element P to element W. Explain. (2 marks)
(ii) What types of oxides are formed by elements:
(i) R …………………………………….. (ii) U …………………………………………. (1 mark) 20 The table below shows the atomic numbers of elements of the periodic table represented by letters A to H. The letters are not the actual symbols of the elements.
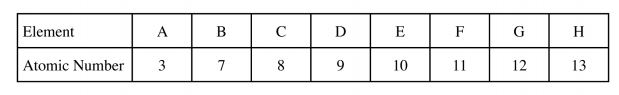
(a) Select from the table two elements that belong to the same group. (1 mark)
(b) Write the formula of a compound formed between elements H and C. (1 mark)
(c) Which of the elements is monoatomic? (1 mark)
(d) Select an element that forms a divalent cation. (1 mark)
21 (a) Identify the types of bonds in carbon (II) oxide molecule. (1 mark)
(b) Explain why graphite is used as a lubricant. (2 marks)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this Section in the spaces provided.
22 A stopwatch showed a reading of 0.05 s before the start button was pressed. When this stopwatch was used to measure the time taken by a stone to drop from the top of a building to the ground it recorded 3.68 s. Determine the time of fall. (2 marks)
23 Define the term density. . ( 1 mark)
24 An object is found to have a weight of 12.5 N at a place where the acceleration due to gravity is 10 ms’2. Determine the mass of the object. (3 marks)
25 Explain how a drinking straw is used to draw a liquid from a bottle into the mouth. (3 marks)
26 State the reason why it is possible to compress a gas but not a liquid. (1 mark)
27 (a) Overhead power cables between posts are allowed to sag a bit. Explain why this is necessary. (2 marks)
(b) Figure 1 shows a bimetallic strip made of brass and iron.
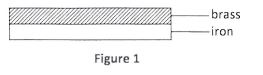
In the space below, draw the appearance of the strip when heated (brass expands more than iron). (1 mark)
28 Define the term heat energy. (2 marks)
29 Figure 2 shows a uniform metre rule pivoted at the 20 cm mark and balanced by a weight of 4.5 N.
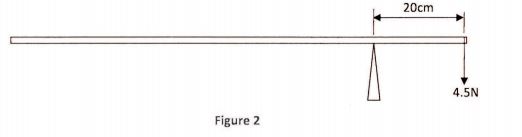
Determine the weight of the rule. (3 marks)
30 Figure 3 shows a cone resting on a flat surface.
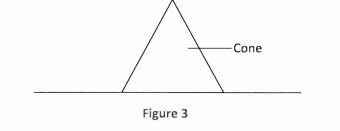
(a) Name the state of equilibrium of the cone. (1 mark)
(b) State the reason for the answer in (a) above. (1 mark)
31 Figure 4 shows a velocity – time graph for the motion of a certain body.
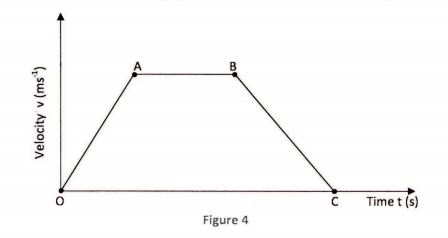
Describe the motion of the body in the region:
(a) OA; (1 mark)
(b) AB; (1 mark)
(c) BC. (1 mark)
32 The length of a spring is 20 cm. When it is used to support a mass of 0.4 kg. its new length is 20.8 cm. Determine the spring constant. (take acceleration due to gravity g =10 ms2 ) (3 marks)
33 It is observed that a passenger standing in a stationary truck tends to fall in a direction opposite to the motion of the truck when it suddenly starts moving. Explain this observation. (2 marks)
34 Figure 5 shows a cork floating in water and held in position by a thin thread attached to the bottom of the container.
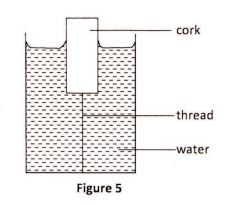
Name three forces acting on the cork. (3 marks)
35 State the energy changes that take place when a torch is switched on starting with the chemical energy in the cells. (2 marks)
3.7.2 General Science Paper 2 (237/2)
SECTION A: BIOLOGY (34 marks)
Answer ALL the questions in this section in the spaces provided.
1 Differentiate between ecology and ecosystem. (2 marks)
2 (a) Name three air pollutants produced when charcoal is burnt in a poorly ventilated room. (3 marks)
(b) Name the causative agent of amoebic dysentery. (1 mark)
3 The diagram below represents the human male reproductive system.
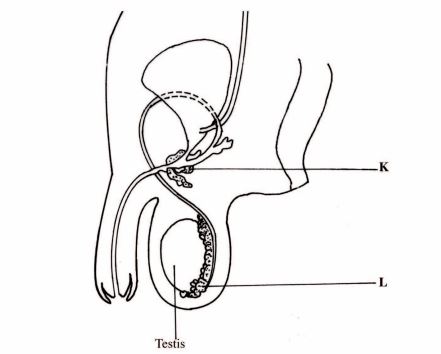
(a) (i) Name the parts labelled K and L.
K; (1 mark)
L. (1 mark)
(ii) State the role of the hormone produced by the testis. (1 mark)
What is meant by the term mitosis? (1 mark)
4 (a)What is gestation period? (1 mark)
(b) State two symptoms of Herpes simplex. (2 marks)
(c) What is a genotype? (1 mark)
5 (a) State the meaning of seed viability.(1 marks) (b) State two reasons why water is required for seed germination.(2 marks)
6 (a) Giving an example, describe continuous growth in animals.(2 marks)
(b) Distinguish between the terms homozygosity and heterozygosity.(2 marks)
7 (a) What is chemical evolution?(2 marks)
(b) State two ways in which meiosis is important in sexual reproduction.(2 marks)
8 State the meaning of the following terms:(3 marks)
(a) irritability;
(b)stimulus ;
(c) response.
9 Name three structures of the human ear that are involved in balance and posture.(3 marks)
10 State three functions of an endoskeleton.(3 marks)
SECTION B: CHEMISTRY (33 marks)
Answer ALL the questions in this section in the spaces provided.
11 The set-up shown below was used to investigate some properties of chlorine gas.
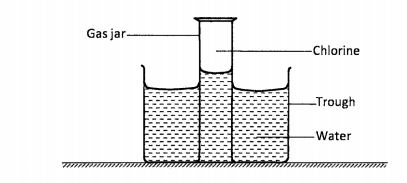
(a) Explain Why the level ofwater in the gas jar was higher than in the trough after some time. (1 mark)
(b) (i) What would be observed if blue litmus paper was dipped into the water in the trough?(1 mark)
(ii) Explain the observations made in b(i) above.(2 marks)
12 Calculate the number of moles contained in 30g of potassium nitrate
(K= 39.0; N = 14.0; O = 16.0). (2 marks)
13 A balloon filled with air was tied and held above a trough containing hot Water as shown in the diagram.
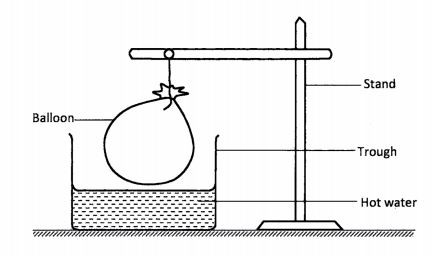
(a) State the observation made on the balloon. (1 mark)
(b) Explain the observation in (a) above. (2 marks)
14 (a) What is meant by the term dilution? (1 mark)
(b) Calculate the mass in grams contained in 25.0 cm} of0.2M sodium hydroxide solution (Na = 23.0; O = 16.0; H = 1.0). (2 marks)
15 (a) Name one natural polymer and state its use. (1 mark)
Natural polymer.
Use.
(b) State one advantage and one disadvantage of synthetic polymers. (1 mark)
Advantage ………………………………………. ..
Disadvantage ……………………………………. ..
16 (a) Iron metal exists naturally in different ores. Other than haematite, name another common ore of iron. (1 mark)
(b) During the extraction of iron metal, one of the reactions in the blast furnace is:
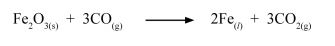
(i) Name the raW material that is used to produce carbon (Il) oxide. (1 mark)
(ii) Iron metal produced in the reaction is in liquid state. Explain.
(c) State with a reason, one use of stainless steel. (2 marks)
Use: …………………………
Reason: ………………………..
17 The set-up shown below was used by a student to prepare sulphur (IV) oxide gas. Study it and answer the questions that follow.
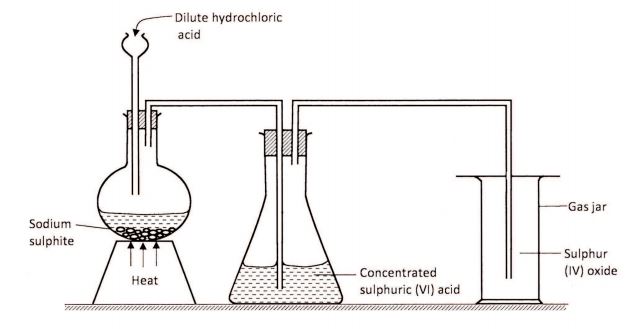
(a) (i) Identify a mistake on the set-up that will affect collection of sulphur (IV) oxide gas. (% mark)
(ii) How would the mistake be corrected? (% mark)
(b) (i) State the use of concentrated sulphuric (Vl) acid in the above set-up. (1 mark)
(ii) What would happen if concentrated sulphuric (V1) acid was replaced with water? (1 mark)
(c) State one use of sulphur (IV) oxide gas. (1 mark)
18 When Potassium chloride was dissolved in water, the following change occurred.
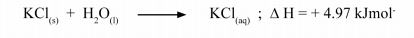
(a) (i) State the type of energy change in the above reaction. (1 mark)
(ii) The above experiment was done in a boiling tube. State the observation that was made. (1 mark)
(iii) Name the type of reaction in a(ii) above.(1 mark)
(b) Name two factors considered when choosing a fuel. (2 marks)
19 (a) Name the compound CH3CHCHCH3 (1 mark)
(b) Name the type of reaction that takes place when the compound in (a) above is reacted with hydrogen chloride gas. (1 mark)
20 0.1M hydrochloric acid was reacted with sodium thiosulphate solution. The time taken for the cross to disappear was recorded at different temperatures as shown on the graph.
(a) Explain the shape of the curve. (1 mark)
(b) What conclusion would be made from the curve? (1 mark)
(c) Sketch another curve on the same axis that would be obtained when the concentration of hydrochloric acid is doubled. (1 mark)
SECTION C: PHYSICS (33 marks)
Answer ALL the questions in this section in the spaees provided.
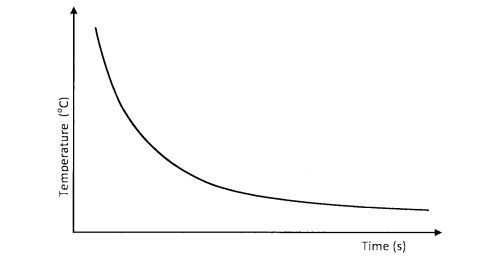
21 Figure 1, shows an image I formed when an object O is placed infront of a convex mirror.
Complete the ray diagram to show the position of object O. (3 marks)
22 When a polythene rod is rubbed with a dry piece of cloth, and then brought near 21 negatively charged pith ball. the ball is observed to move away. Explain this observation. (2 marks)
23 (a) Name one defect of a simple cell. (1 mark)
(b) State how the defect in (a) above is minimized. (1 mark)
24 Figure 2 shows iron keepers used in storing bar magnets.
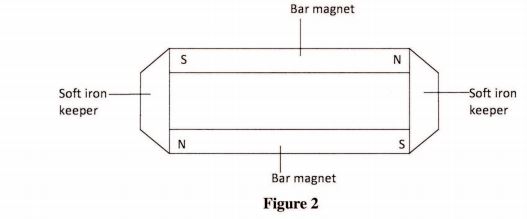
On the figure show the poles induced in the keepers. (1 mark)
25 Figure 3, shows an arrow which indicates the direction of travel of a wave in a medium. P, a particle of the medium is in the path of the wave.

In the space provided, sketch the diagram to show how the particle P moves when the wave is (a) transverse.
(b) longitudinal (2 marks)
26 State two factors that affect the speed of sound in air. (2 marks)
27 Define the term potential difference. (1 mark)
28 Figure 4, shows two circuits X and Y in which two identical coils are used to heat two equal amounts of water. The two circuits are switched on at the same time.
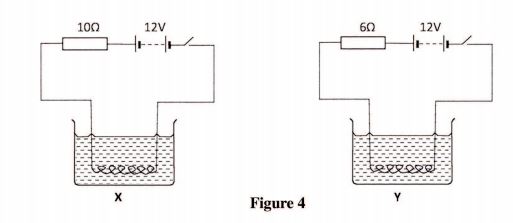
(a) State the circuit in which the water boils first. (1 mark)
(b) Explain the answer in (a) above. (2 marks)
29 It is observed that a swimming pool full of water appears shallower than it actually is. Explain this observation. (3 marks)
30 Figure 5, shows an object O placed in front of a converging lens whose principal foci are F1 and F2.
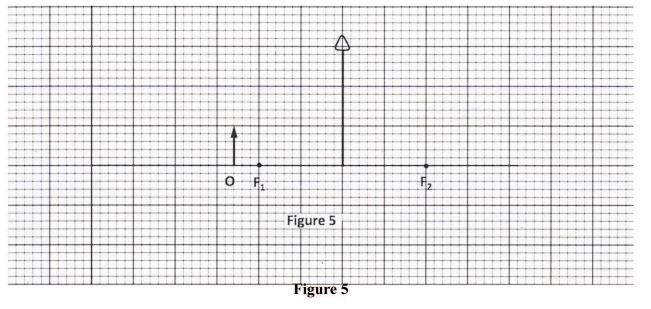
Using rays, complete the diagram to show the position of the image. (3 marks)
31 Figure 6, shows a displacement – time graph of a wave.
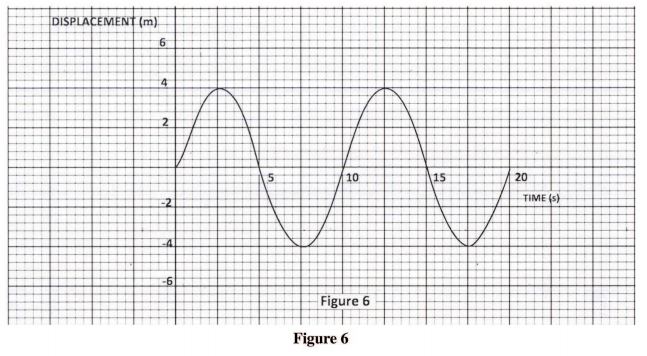
Determine the amplitude of the waveform. (1 mark) 32 An electric iron is rated 1500 W. Determine the cost of using the iron for 30 hours given that the cost of electricity is Ksh.8 per kilowatt hour. (3 marks)
33 (a) State one way in which the path of a cathode ray can he changed. (1 mark)
(b) The control grid in a cathode ray oscilloscope (CRO) is used to control the brightness of the spot on the screen. Explain how the brightness of the spot may be reduced. (2 marks)
34 State two ways in which the conductivity of a semiconductor can be increased. (2 marks)
35 Explain the danger of radioactive emissions on a human body. (2 marks)
2014 KCSE General Science Past Paper-Marking Scheme/Answers
4.7 GENERAL SCIENCE (237)
4.7.1 General Science Paper 1 (237/1)
SECTION A : BIOLOGY
1. (a) Avoid spilling chemicals / acids which corrode bench tops;
Avoid cutting specimens on bench tops because they scratch bench surfaces;
Avoid placing hot objects on bench tops Which may burn the tops.
(1 mark)
(b) Distribution of nutrients / transport of nutrients only;
Availability of raw materials for photosynthesis;
Cooling effect to the plant;
(3 marks)
2. (a) Dicotyledonae; (1 mark)
(b) Leaves have net venation;
Have tap root system;
(1 mark)
3. (a) There was movement of Water molecules from the beaker into the strip of pawpaw by osmosis; because the strip tissue fluid Was hypertonic / Water in the beaker was hypotonic; (2 marks)
(b) The strip became rigid / hard / stiff / turgid / firm;
The strip bent / curved;
(1 mark)
4. (a) Peripheral nucleus; Large central vacuole;
Cell Wall present; Has regular shape ; /chloroplast present (3 marks)
(b) Chloroplast; (1 mark)
5. (a) (i) Control reaction rates; (1 mark)
(ii) They are protein in nature; (1 mark)
(b) In low temperature the rate of photosynthesis is slow / rate increased With increase in temperature upto to optimum; beyond optimum temperature, rate decreases and eventually photosynthesis stops;
6. To prevent the spread of immunizable diseases; (a) Enables the individual to develop immunity/to boost/increase immune system; against immunizable infections; (2 marks) (b) Unusual response by the body cells to a foreign substance; (1 mark) 7. (a) Metabolism is slower in older people; because of low oxygen demand; leading to lower rate of breathing. (vice versa)(2 marks) (b) Micobacterium tuberculosis,”(1 mark) 8. (i) To find out if heat if produced by germinating seeds; as they respire.(1 mark) (ii) To kill bacteria that would respire producing heat;(1 mark) (b) Glucose; Amino acids;(2 marks) 9. Excretion of excess salts / water / waste products;(2 marks) Cooling of the body; by evaporating sweat.(2 marks) (b) Excess water collects in contractile vacuoles; which discharge the contents to the exterior;(2 marks) 10. Liver has many metabolic activities which release heat; which is distributed by blood to maintain body temperature; When it is cold the liver metabolic activities increase / when it is hot the liver metabolic activities decrease;(3 marks) SECTION B : CHEMISTRY (33 marks)
11. (a) (i) – White (½) anydrous copper (II) Sulphate tums blue(½) (1 mark)
(ii) – Anhydrous copper (II) Sulphate combines with the Water produced to form blue hydrated copper (II) Sulphate. \/ (1) / anhydrous Copper (II) Sulphate becomes hydrated. (1 mark)
(b) It causes global warming / greenhouse effect. \/(1) (1 mark)
12. (a) Q – Manganese (IV) oxide (MnO2). \/(1) (1 mark) MnO (b) ZHZOZWUM ZHZOU) + OM) \/(1) (1 mark)
Catalyst Penalise % mark for absence or
Wrong symbols
(c) Sodium peroxide and water
OR
Na2O2 and H20
(1 mark)
(d) Oxygen relights / rekindles a glowing splint. \/(1) (1 mark)
13. (a) Solution On/(1) (1 mark)
(b) Salt / sodium salt, carbon (IV) oxide and water \/ <1) (1 mark)
1 mark – 3 correct
1/2 mark – 2 correct
O mark – 1 correct
14. (a) (i) Hygroscopic salt refers to a salt that absorbs Water from the atmosphere but does not form a solution. 1/<1) (1 mark)
(ii) KNO3, anhydrous copper (II) sulphate (CuSO4)/anhydrous magnesium chloride and anhydrous calcium chloride. \/ (1) (1 mark)
(b) – Manufacture of chlorine
– Manufacture of Sodium carbonate \/ (1)
– Extraction of sodium metal
(1 mark)
15. (a) Absence of source of heat \/ (1) / solid PbBr, instead of molten lead (II) bromide. _ (1 mark)
(b) Bromine gas / fumes \/<1) (1 mark)
16. (a) – It provides calcium and magnesium ions ‘/(72) used by animals in formation J (%) of strong bones, teeth and shells.(1 mark) (b) Hard water is passed J (1)through a column packed with a compound of sodium permuttit which exchanges Ca“ J (1) and Mg“ ions for Nat ions. (2 marks) 17. (a) Red and blue J(1)(1 mark) (b) Brown ink J(1)(1 mark) 18. – Add water to the mixture, sodium chloride dissolves J (1) – Filter to obtain sulphur J(1) – Evaporate filtrate to obtain sodium chloride. J (1) (3 marks) 19. (a) Ionization energy is the minimum energy required to completely remove the outermost electron from an atom in the gaseous state. J (1) (w.t.t.e) (1 mark) (b) (i) The atomic size decrease from P to W because of increase in the effective nuclear charge J (1) hence the removal of the outermost electron across the period requires more energy J (1) as the atomic size decreases. (ii) (I) R – Amphoteric oxide J (%) (II) U – Acidic oxide J <%) 20. (a) A J(%) andF \/(V2) (b) HZC3 t/<1) OR A1203 (C) E J(1) (<1) G t/<1) 21. (a) – Covalent bonds J (72) – Coordinate/dative bond J (%) (2 marks) (1 mark) (1 mark) (1 mark) (1 mark) (1 mark) (J (%) mark for the structure only) (1 mark) (b) Graphite exist in hexagonal layers which are held together by weak van der waals forces of attraction J (1). These layers slip/slide over each other when compressed hence this makes it a good lubricant. J (1) (2 marks) SECTION C : PHYSICS 22. Time of fall = 3.68 — 0.05 ; 1 : 3.63s ; l will _Q 23. Mass per unit volume/density v01um6/ _ V /8_ V ; l 24. W = mg ; 1 12.5 = m >< 10 , 1 m = l.25kg , 1 25. On sucking at the open end of the straw, the air inside is removed/sucked out; l This lowers/reduces/decreases the pressure inside the straw; l The atmospheric pressure now being higher than the pressure inside the straw; l forces the liquid up the straw. 26. The intermolecular distances in gases are larger/greater/higher/1arger/more than/bigger than in liquids; I 27. (a) This is to allow for contraction ; during cold weather, l If this is not done the wires would break ; causing alot of damage. l (b) ’ / / / –‘/, J7 ; “/0 K B ra ss % I ron 28. Heat is energy transferred from place to place; 1 29. Sum of anticlockwise moments = Sum of clockwise moments/Fldl = Fzd as a result of temperature difference; l Form of energy; when absorbed/taken in, temperature rises; or when lost/ given out, temperature f— Let weight of rule be x ; l x >< 30 = 20 >< 4.5 ; l x = 20 X 4.5 30 = 3 N , 1 30. (a) (b) 31. (a) (b) (C) Stable ; 1 If the cone is slightly displaced, the position of the centre of gravity rises and falls /broad base/larger base/bigger base; 1 back to he same position When the displacing force is removed/vertical line passing through the c.o.g. falls within the base. The body undergoes uniform/steady/constant acceleration /velocity increasing at uniform rate; 1 The body moves with constant/steady/uniform velocity; 1 zero acceleration. The body moves with a uniform/steady/constant deceleration / velocity decreasing at uniform rate; 1 uniform negative acceleration. 32. Extension = 20.8 – 20 = 0.8 cm; 1 F = Ke 400 _ 0.8 1000*“) _KX100 * 1 K: O8 = 500 Nm”; 1 4 >< 100 33. This is caused by the tendency of the body to remain stationery (inertia); 1 since the body Was initially at rest, it tends ; 1 to remain at rest as the truck moves forward. 34. – Weight/mg/gravitational ; 1 – Upthrust ; 1 – Tension ; 1 35. Chemical to electrical , 1 Electrical to light / heat , 1 4.7.2 General Science Paper 2 (237/2)
SECTION A: BIOLOGY
1. Ecology is the study of interaction of organisms with one another and with their physical environment while Ecosystem is the sum total of the interactions linking organisms in a community with one another and with their environment. (2 marks)
2. (a) Carbon (II) Oxide;
Carbon (IV) Oxide;
Carbon particles / soot;
(3 marks)
(b) Entamoeba histolytica; (1 mark)
3. (a) (i) K – Prostate gland; L – Epididymis; (2 marks)
(ii) Controls development of secondary sexual characteristics;
leads to formation of spermatozoa/sperms; (1 mark)
(b) It is a type of cell division giving rise to two identical diploid daughter cells; (1 mark)
4. (a) The time between implantation and birth.
(1 mark)
(b) Genital sores /painless sores;
Ulcers in affected areas;
(2 marks)
(c) It is the genetic constitution / make up of an organisms; (1 mark)
5. (a) Ability of a seed to germinate; (1 mark)
(b) It softens the seed coat;
It hydrolyses stored food;
It is a medium for enzyme activity / activates enzymes / transports dissolved nutrients; (2 marks)
10 . (a) (b) . (a) (b) . (a) (b) (C) In continuous growth, the animal grows all the way from fertilization till maturity; e.g. height in humans; (2 marks) Homozygosity is a condition in which the gene pairs are similar / same / identical while Heterozygosity is a condition in which the gene pairs are different. (2 marks) It is a theory on the origin of life; that suggests that life began from simple elements through complex compounds; (2 marks) Meiosis leads to the formation of the gametes which are haploid; Meiosis ensures that the chromosomal constitution of offspring is the same as that of parents; (2 marks) Irritability – ability to detect and respond to changes in the environment; (1 mark) Stimulus – A detectable change in the environment capable of producing a response; ( l mark) Response – the change in activity by the organism due to a stimulus. (1 mark) Semicircular canals; Utriculus; Sacculus; (3 marks) . Give the body its shape; Protects delicate intemal organs; Provides surface for attachment of muscles; Produces / manufacture blood cells; Stores calcium and phosphate ions; Enables/allows movement; Gives body balance and posture; Supports the body wight. (3 marks) SECTION B : CHEMISTRY (33 marks) 11. (a) Chlorine gas is slightly soluble in Water \/ (%) hence Water rises to occupy \/(%) the space which Was occupied by the dissolved gas. (l mark) (b) (i) OR Turns red ‘/ lyzlthen bleached */%) decolourised / turned white \/(1) (1 mark) red. \/(1) (11) Chlorine gas dissolves in water to fonn acidic solution which turns blue to The red litmus paper is further decolourised / bleached due to presence of hypochlorous acid or chloric (I) acid. \/ <1) 12. Formula mass of KNO3\/(M2) : Number of moles in 30 g = 39 +14 + 3 (16) 53 +48 101% vw 30/101 vw (2 marks) 4 marks 13. (a) (b) 14. (a) (b) = 0.2970 moles \/(%) (2 marks) 2 marks The balloon increased / expanded in size/volume. \/ (1) (1 mark) Rise in temperature increases kinetic energy of air molecules in the balloon hence increased collisions / bombardments against the Walls of the balloon, \/ (1) causing the balloon to expand. (2 marks) 3 marks A process Where a solvent is increased/Water is added/increased \/ (l)while the amount of solute remains constant in a given solution. (1 mark) Formula mass of NaOH = 23 + l6 + 1 = 40 g Moles in 25cm3 = % vw Mass in 25cm3ofNaOH = m >< 40 um 1000 = 0.2 g \/(%) (2 marks) 3 marks ISK 15. (a) Natural polymers Cellulose / proteins / natural rubber/silk/Wool. \/ (%) i Used for paper manufacturing, textiles – Rubber used in tyres, tubes \/(%) – Proteins / cellulose in textiles. (1 mark) (b) Advantages of synthetic polymer – Cheap / long lasting / moulded into many shapes. \/ (72) – Prevent / safe destruction of plants and animals. – Some are heat resistant / good insulators / non corrosive to acids / alkalis. (any one correct for (V1) mark) (ii) Disadvantage of synthetic polymer \/(W – Pollutants to the environment \/<%) – Non-biodegradable – Costly to recycle – Burn producing poisonous gases. (1 mark) (any one correct at (%) mark) 2 marks 16. (a) Magnetite / Pyrite. J (1) OR FeCO3 / FeS (l mark) (b) (i) Coke /Carbon \/ (1) (l mark) (ii) Temperatures at the blast furnace are higher than the melting point of iron metal. \/ (1) (1 mark) (c) Use of stainless steel – Construction of bridges – In ships – Pipes, padlocks – Nails – Cutlery. (1 mark) (any one correct for 1 mark) Reason: Stainless steel does not rust /is resistant to corrosion. (1 mark) 5 marks 17. (a) (b) (C) 18. (a) (b) 19. (a) (b) 20. (a) (i) The thistle funnel is above the reagents and allows the gas to escape \/ (M2) / OR The thistle funnel doesn‘t have a tap. (% mark) (ii) Use a dropping funnel / Dip the thistle funnel into the reagents. \/ M) (i) Used to dry the sulphur (IV) oxide/as a drying agent. (/ (1) (% mark) (ii) The sulphur (IV) oxide gas would dissolve in the water, hence no gas would be collected in the gas jar. Uses of sulphur (IV) oxide – Manufacture of sulphuric (VI) acid (/(1) / sulphuric acid/ HZSO – Bleaching agent – As a refrigerant – Fruit preservative – lnfumigation/to kill germs (any one correct for 1 mark) (i) Heat / enthalpy of solution \/ (1) (ii) The temperature would drop / boiling tube becomes cold. (/ (1) (m) Endothermic reaction. \/(1) – Heat value \/(1) – Cost of fuel \/(1) – Availability – Environmentally friendly/less pollution – Cost of transporting the fuel/toxicity of fuel (Any two correct for 2 marks) But – 2 – ene \/(1) Addition reaction (/ (1) 4 (1) ( l mark) 4 marks (1 mark) (1 mark) (l mark) (2 marks) 5 marks (1 mark) (l mark) 2 marks As the temperature rises, the time taken for the cross to disappear decreases. \/ (1) OR as the temperature decreases, the time taken for the cross to disappear increases. ( l mark) (b) The rate of reaction increases with rise in temperature. \/ (1) OR The rate of reaction decreases With decrease in temperature. (c) Diagram. Temnerature \ \ \ G \ Q» \ \ \ \\ ofacid \ \\\ (1 mark) High concentration _ P Time (S) § 1 mark} i SECTION C : PHYSICS 21. / 5 / ; 1 ~> \ .1 \\\\ :1 / A7/’ / / / \ \\\ \ ~\\ \ ~__ \ O —n rw 22. When the polythene rod is rubbed it acquires a negative charge; On bringing it near. l a negatively charged pith ball the two repel one another; l 23. (a) Polarization / local action/polarization/accummulation of hydrogen bubbles around the cathode l (b) Polarization is minimised by adding depolarizer /pottasium dichromate/ local action/ dissolving of zinc/Zinc being eaten / local action is minimised by coating zinc with mercury/amalgamation/using pure zinc. l 24. S NS keeper keeper Pm? N S 25. (a) M t‘ fP ; 1 . . . O Ion O Perpendicular oscilation. (b) Mmlo” °l P l 1 Parallel along direction of the wave. 26. – Temperature; 1 – Humidity; 1 – Density of air 29 27. Work done in moving a unit charge from one point to another; 28. (a) Circuit Y; 1 (b) More current is flowing due to less; 1 resistance in the circuit hence a greater heating effect; – The ray of light from the bottom is bend away from the normal; – The image of the bottom appears raised; 30. -1 -1 >-1 . – Light rays from the bottom of the pool are refracted at the interface; : I F1 F 31. a=4m; 1 32. 1500 + 1000 = 1.5 kW; 1 Cost of electricity = 1.5 x 3O x 8; 1 : Ksh.36O ; 1 33. (a) By using a magnetic or electric field ; 1 (b) – By increasing the grid potential so that/making the grid more negative; 1 fewer electrons reach the screen/more electrons are repelled 34. – By raising the temperature of the semiconductor; 1 – By doping the semiconductor ; 1 35. – Radiations have high energy ; 1 which damages the body cells; 1 ; 1

