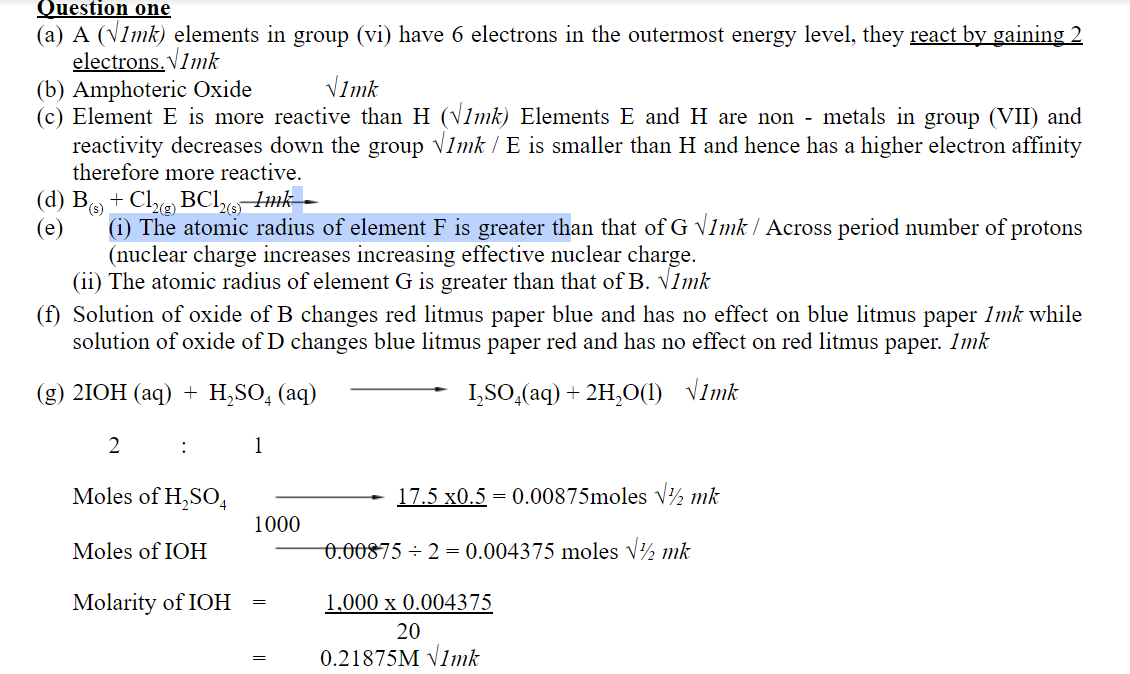
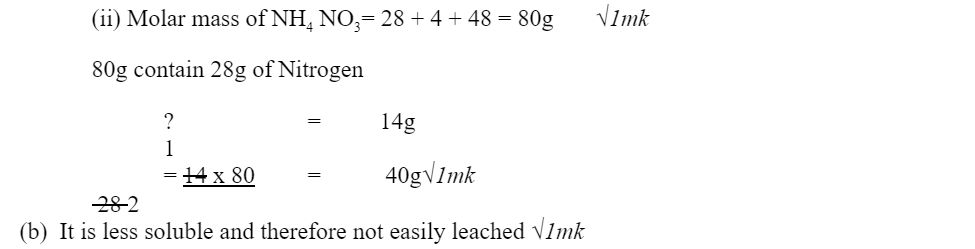1.The grid below shows part of the periodic table. Study it and answer the questions that follow. The letters do not represent the true symbols of the elements.
| A | ||||||||
| I | B | C | D | E | ||||
| F | G | H | ||||||
a) Which element forms an ion of charge – 2? Explain your answer 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) What is the nature of the oxide formed by element C? 1mark
………………………………………………………………………………………………………………
c) How does the reactivity of H compare with that of E? Explain. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
d) Write the chemical equation for the reaction between B and chlorine? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
e) Explain how the atomic radii of the following compare; 2marks
i) F and G
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii) B and G
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
f) The oxides of B and D are separately dissolved in water. State the effect of each product on litmus paper. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
g) 20cm3 of a solution of a hydroxide of I completely neutralizes 17.5cm3 of 0.5M sulphuric (VI) acid. Calculate the concentration in moles/litre of solution of the hydroxide of I 3marks
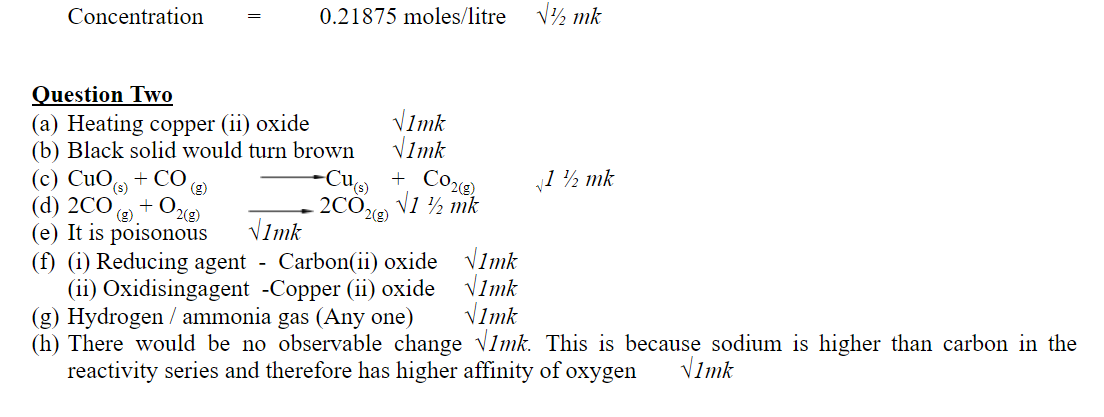
2. The diagram below shows an experiment set-up to investigate a property of carbon (ii) oxide. Study it and answer the questions that follow.
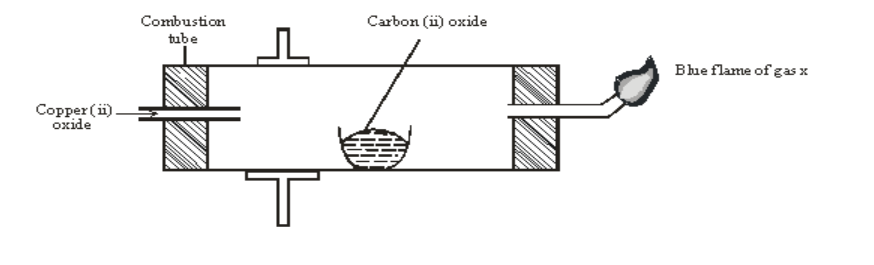
a) Name one condition that is missing in the set up that must be present if the experiment to proceed. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b)If the experiment was carried out properly. What observation would be made in the combustion tube? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
c) Give an equation for the reaction that occurs in the combustion tube. 1 ½ mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
d) Give an equation for the reaction that takes place as gas x burns. 1 ½ marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
e) Why is it necessary to burn gas x? 1mk
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
f) Name the reducing and oxidizing agent. 2marks
i)Reducing agent
………………………………………………………………………………………………………………
ii)Oxidising agent
………………………………………………………………………………………………………………
g) Identify any other substance that would have the same effect on copper (ii) oxide as carbon (ii) oxide. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
h) What would happen if copper (ii) oxide was replaced with sodium oxide? Explain 2mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
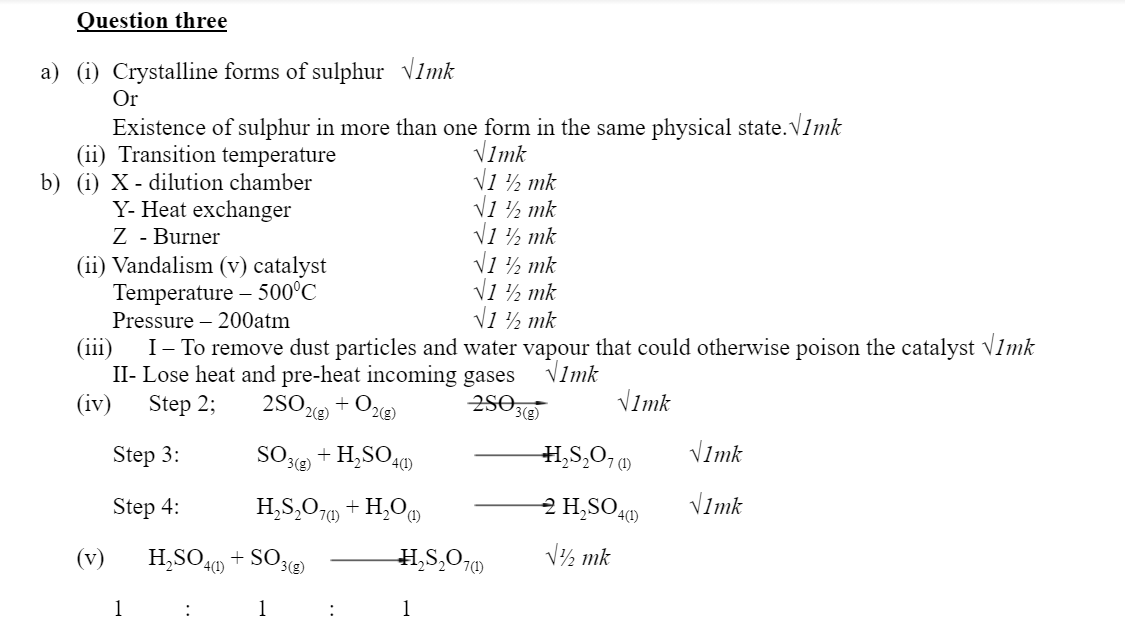
3.a) Sulphur occurs naturally in two different forms called allotropes;
i) What are allotropes? 1mark
………………………………………………………………………………………………………………
ii) The two allotropes of sulphur are stable at different temperatures, as shown in the equation below.
Above 95.50C
Rhombic sulphur ————→ Monoclinic sulphur
Below 95.50C
Give a name to the temperature 95.50C 1mark
………………………………………………………………………………………………………………
b) Below is a flow chart diagram for the contact process for the manufacture of sulphuric (VI) acid.

i) Give the name of chambers labeled 1 ½ mark
X
………………………………………………………………
Y
………………………………………………………………
Z
……………………………………………………………….
ii) State the three conditions in the converter. 1 ½ mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
iii) Explain why gases are passed through ; 2marks
I – The dust precipitator and drying power
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
II- The chamber labeled Y
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
iv) Write the balanced equations for the reactions in; 3marks
Step 2:
………………………………………………………………………………………………………………
Step 3:
………………………………………………………………………………………………………………
Step 4:
………………………………………………………………………………………………………………
c) Calculate the volume of sulphur (VI) oxide gas in litres that would be required to produce 178kg of Oleum in step 3. (Molar gas volume at s.t.p.=22.4l, H=1, O=16, S=32) 3marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
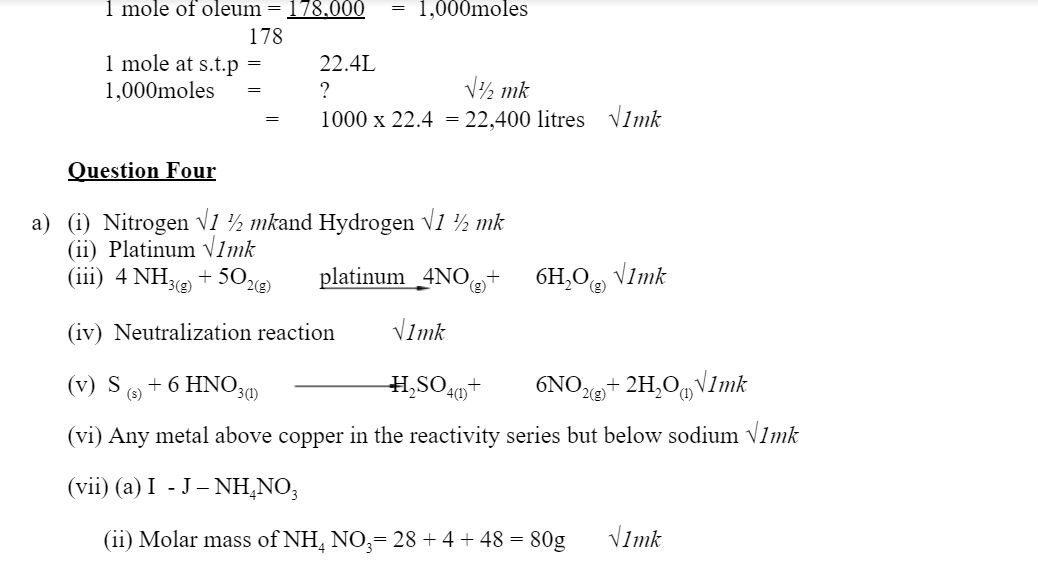
4. a) The scheme below shows various reactions starting with ammonia. Study it and answer the questions that follow.
i) List the raw materials used in the manufacturer of ammonia gas. 1mark
……………………………………………………………………………………………………………..
ii) What catalyst is used in step I? 1mark
…………………………………………………………………………………………………………………………………………………..
ii)Write an equation for the reaction that occurs between ammonia and oxygen gas in the presence of a catalyst. 1mark
…………………………………………………………………………………………………………………………………………………..
iv) Identify the process is step II? 1mark
………………………………………………………………………………………………………………
v) Using an appropriate equation, explain how the reaction in step III occurs (1 mark)
………………………………………………………………………………………………………………
(vi) What should be added to solution K to form solid L? (1 mark)
………………………………………………………………………………………………………………
(a) I. Write the formula of compound J.
………………………………………………………………………………………………………………
II. Calculate the mass of compound J that would contain 14g of nitrogen. (N=14, O=16, H= 1) (2marks)
b) Explain the advantage of using ammonium phosphate fertilizer over the other nitrogenous fertilizers. (1mark)
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
5. Dry chlorine was collected using the set up below.
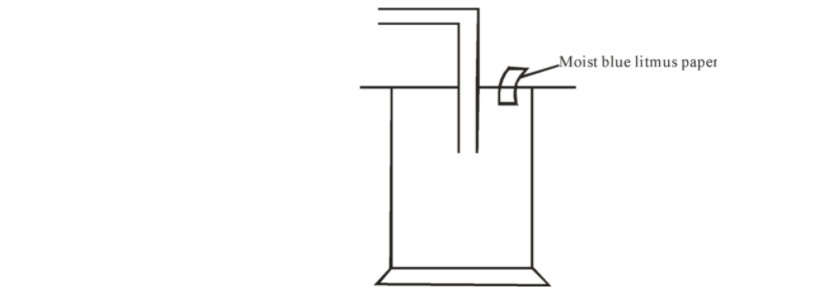
a) Name a suitable drying agent for chlorine gas? 1mark
…………………………………………………………………………………………………………….
…………………………………………………………………………………………………………….
b) State one property of chlorine gas which facilitates this method of collection. 1mark
…………………………………………………………………………………………………………….
…………………………………………………………………………………………………………….
c) State the observations on the moist blue litmus paper. 2marks
………………………………………………………………………………………………………….…..
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
d) Chlorine gas was bubbled through distilled water. With aid of an equation show the formation of chlorine water. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………..…….
………………………………………………………………………………………………………………
e) Write the formula of the compounds formed when chlorine gas reacts with warm dry phosphorous. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
f) Chlorine gas is mixed with moist hydrogen sulphide gas, state and explain the observations 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………
g) Give one use of chlorine gas. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
6.Fractional distillation of air is used in the industrial manufacture of oxygen. The diagram below shows the process.
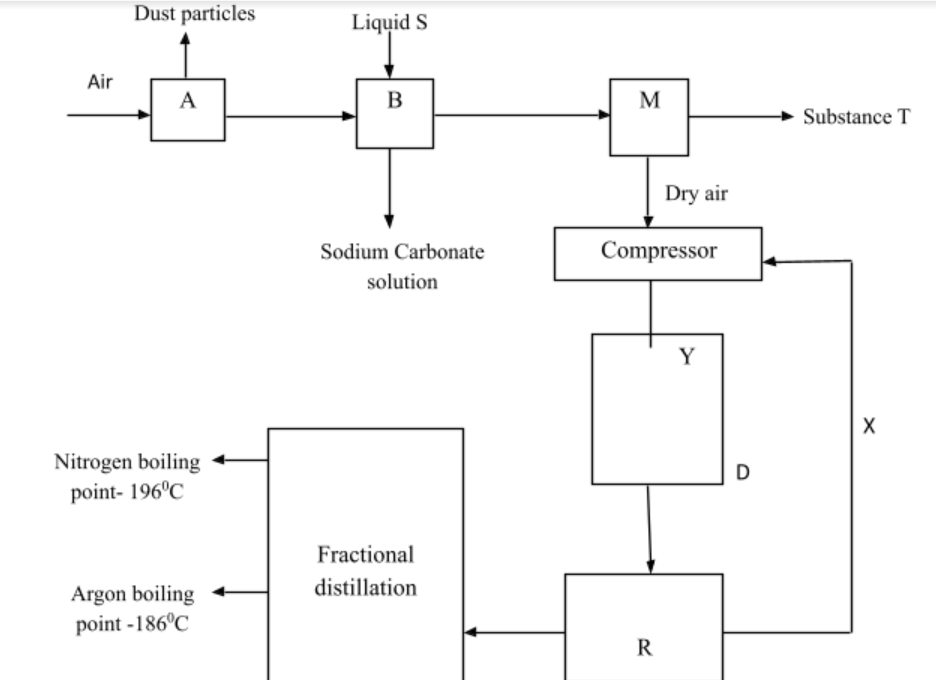
What processes are taking place in chamber A,B,M and D 2marks
A …………………………………………………………………………………………..……..…………..
B …………………………………………………………………………………………….………………..
M ……………………………………………………………………………………………..…….………….
D …………………………………………………………………………….………………..……………….
a)Name;
Liquid S
……………………………………………………………………………………….……….……………
Substance T
…………………………………………………………………………….…….…………………………
c) Explain why part Y in chamber D is curved? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
d) Give two industrial uses of oxygen gas? 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
e) In the laboratory preparation of oxygen, manganese (iv) oxide and hydrogen peroxide are used. Write an equation to show how oxygen gas is formed. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
f) An investigation was carried out using the set-up below. Study it and answer the questions that follow.
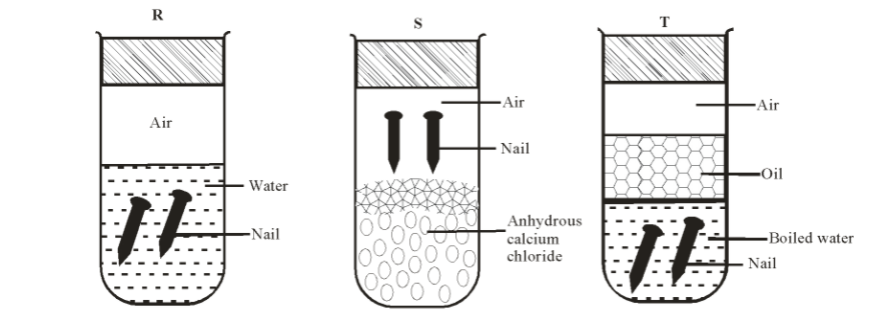
i) State and explain what will happen in the three test-tubes R, S and T after seven days. 3marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii)Give one reason why some metals are electroplated. 1mark
………………………………………………………………………………………………………………
7.Below is a scheme of some reactions of propanol. Study it and answer the questions that follow.
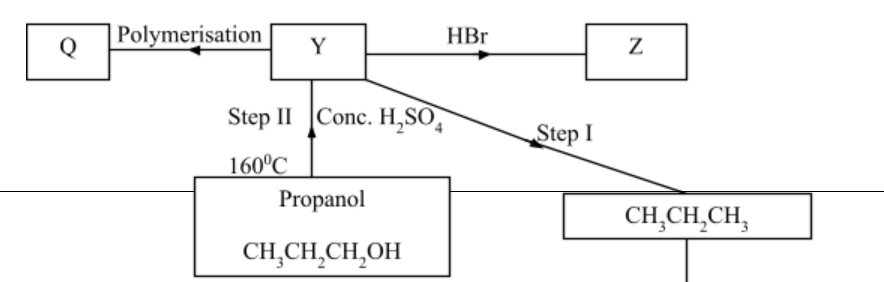
a) State the reagents and conditions required to effect step I 3marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
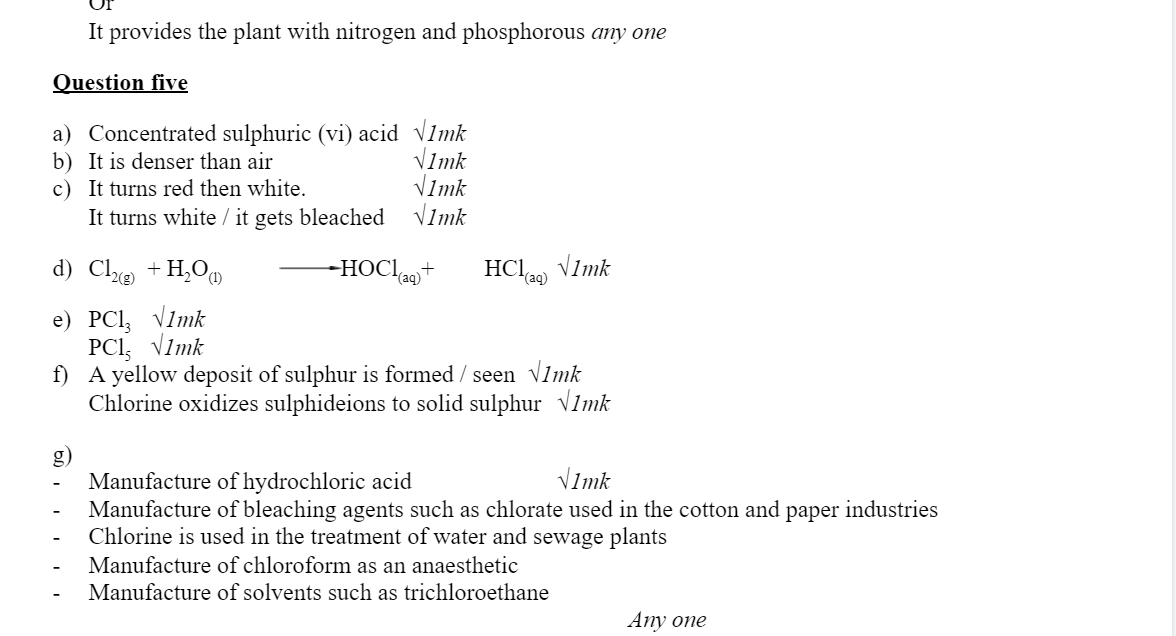
b) Draw the structural formulae and name product Z. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
c) Name product Q 1mark
………………………………………………………………………………………………………………
d) Explain how product Y can be distinguished from the product formed after step I has taken place. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………..
e) What name is given to the process in Step II and step III 2marks
Step II
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
Step III
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
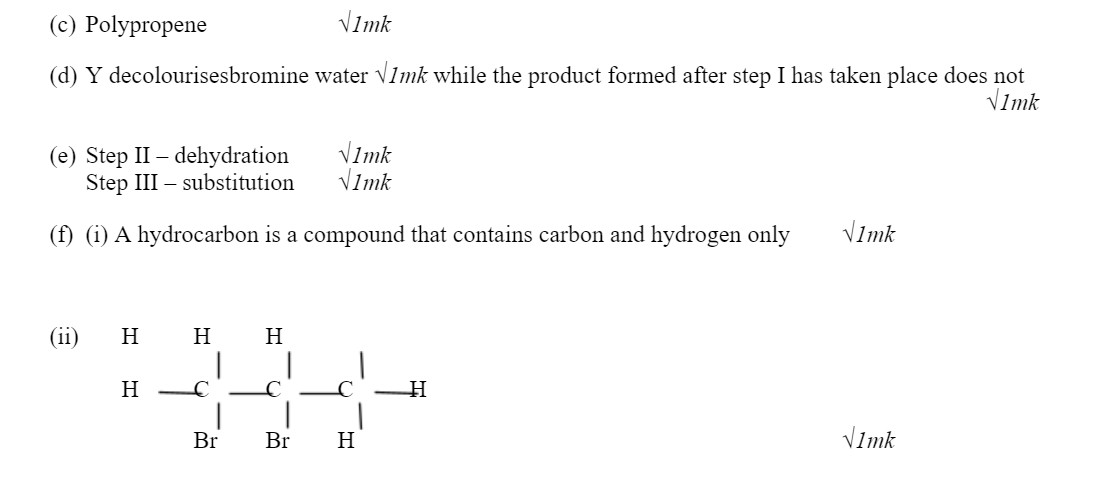
f.(i) Define the term hydrocarbon 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Draw the structure of 1, 2 – dibromopropane 1mark
Marking Scheme