Questions
1. Solution A is prepared by dissolving 6.3g of the organic acid H2C2O4.nH2O in water to make a litre of the solution.
Solution B: 0.1M NaOH solution
Phenolphthalein indicator
Clamp and stand
Burette and pipette.
You are required to determine the value of n in the organic acid H2C2O4. nH2O Procedure.
Fill the burette with solution A and adjust the volume to zero mark.
Add 2 to 3 drops of phenolphthalein indicator and titrate solution A against solution B until the colour just permanently changes.
Record your results in the table below.
Repeat the procedure two more times to obtain concordant results.
| Titration | 1 | 2 | 3 |
|---|---|---|---|
| Final burette reading (cm3) | |||
| Initial burette reading (cm3) | |||
| Volume of solution A used (cm3) |
4marks
b) Calculate the average volume of solution A used. 1mark
c) Calculate the moles of sodium hydroxide in the volume of solution B used. 2marks
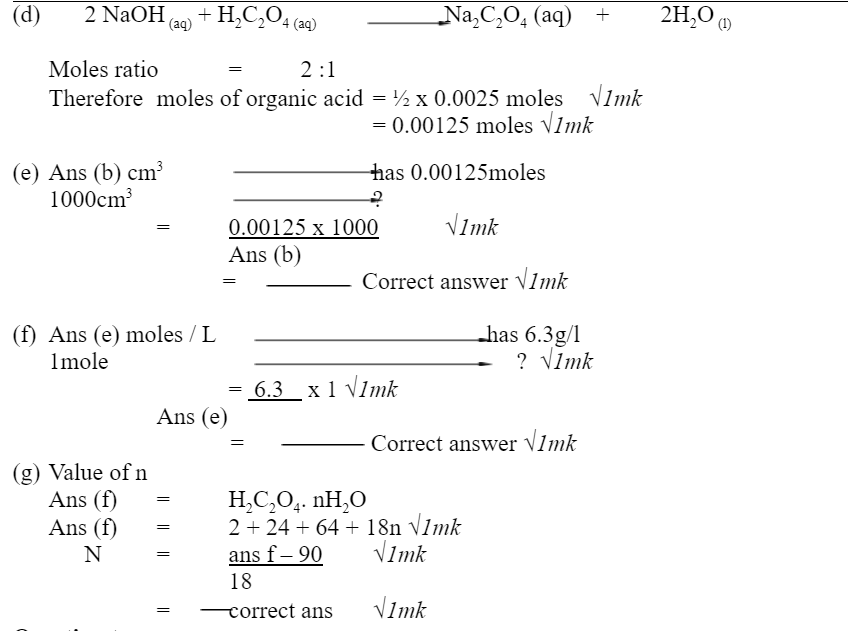
d) Given that solution B – Sodium hydroxide and solution A organic acid react in the ration of 2:1, calculate the number of moles of the organic acid –solution A used? 2marks
e) Calculate the moles of organic acid solution A used per litre of solution 2marks
f) Calculate the relative formula masses of the organic acid solution A 3marks
g) Calculate the value of n in H2C2O4.nH2O(H=1, C=12, O=16) 3marks
2.You are provided with CBI. Carry out the test below. Write your observation and inferences in the spaces provided.
a) Using a clean spatula, heat about one third of the solid CBI in a non- luminous Bunsen burner flame.
| Observation | Inferences |
|---|---|
b) Put a half spatula endful of CBI in a test tube. Heat gently and then strongly.
Test for any gas produced using litmus papers.
| Observation | Inferences |
|---|---|
c) Put 2cm3 of dilute hydrochloric acid into a test tube. Add ¼ endful of CBI into the test tube. Test for any gas procedure.
| Observation | Inferences |
|---|---|
3.You are provided with solid Q, carry out the test below.
Record your observations and inferences in the table. Identify any gas (es) evolved.
Place all the solid Q provided into boiling tube and add distilled water until the tube is ¼ full.
Divide it into five portions.
a) To the 1st portion add ammonia solution drop wise until excess.
| Observation | Inferences |
|---|---|
b) (i) To the 2nd portion add sodium hydroxide solution dropwise until in excess. Keep the resulting mixture for the next test.
| Observation | Inferences |
|---|---|
ii) Warm the preserved mixture from b (i) above
| Observation | Inferences |
|---|---|
c) i) To the 3rd portion add silver nitrate solution. Preserve the mixture for the next test.
| Observation | Inferences |
|---|---|
ii) To the preserved mixture in c (i) above add diluted nitric acid.
| Observation | Inferences |
|---|---|
d) To the 4th portion add dilute Barium nitrate solution followed by dilute nitric acid. To the 5th portion add 2-3 drops of conc. Nitric acid. War
| Observation | Inferences |
|---|---|
e) To the 5th portion add 2-3 drops of conc. Nitric acid.
Warm the mixture and allow to cool. Add sodium hydroxide solution dropwise until in excess.
| Observation | Inferences |
|---|---|
Kenya High Mock Chemistry Paper 3 2021 Answers
Question one
(a) Complete table √1mkComplete table with 3 titres √1mkIncomplete table with 2 titres √½ mkIncomplete table with 1 titre −0 mk
ConditionsPenalize ½ mk for unrealistic values unless where explainedPenalize ½ mk for any inversion of tablePenalize ½ mk for any arithmetic error
NB: penalize a maximum of ½ mk for any of the conditions above.
Decimal √1mk
Award 1mk for 1d.p. or 2 d.p used consistently
If 2d.p used, 2ndd.p. can only be “0” or “5”
Accuracy √1mk
Award 1mk for any value + 0.1 of s.v.
Award ½ mk for any value + 0.2 of s.v.
Award 0mk (penalize fully) for any value beyond + 0.2 of s.v.
Principles of averaging √1mkValues averaged must be consistentIf 3 titres but only 2 are consistent and averaged award 1mkIf 3 titres done and averaged award 1mkIf 3 titres done and inconsistent and averaged award 0mkIf 3 titres done and all are consistent but only 2 are averaged award 0mk
Final answer √1mkAward 1mk for ans. + 0.1 of s.v.Award ½mk for ans. + 0.2 of s.v.Award 0mk ifansnot within + 0.2 of s.v.Marks awarder as follows: CT 1mk
(b) Average titre = t1 + t2 + t3 = (√½ mk)Correct Ans ½ mk
(c) Moles of NAOH = M x V 1000 = 0.1 x 25√1mk = 0.0025moles √1mk
Question 2
| Observation | Inferences |
|---|---|
| Yellow flame √1mk | Na+ ions √1mk |
| Colourless, odourless gas produced Gas turns moist blue litmus paper red Red litmus paper remains red Droplets of colourless liquid on cooler parts of test tube Any 2 x ½ = √1mk | Gas acidic CO 32- , HCO3- ions Hydrated salt / water of crystallization Any 2 correct x ½ = √1mk |
| Effervescence / bubbles Colourless , odourless gas produced Gas turns moist blue litmus paper red Red litmus paper remains red Any 4 x ½ = √2mks | CO 32- , HCO3- ions Gas acidic Any 2 x 1 = √2mks |
Question 3
| Observation | Inferences |
|---|---|
| Pale green ppt√½ mk insoluble in excess √½ mk | Fe2+√1mk |
| Pale green ppt√½ mk Insoluble in excess √½ mk | Fe2+√1mk |
| Gas with pungent . chocking smell 1mk Moist red litmus paper turns to blue 1mk Blue litmus paper remains blue any 2 x 1 = √2mks | Gas basic √½ mk NH4+ ions present√½ mk |
| White ppt | CO 32-, Cl-ions , SO32- |
| White ppt√½ mk Insoluble / persists √½ mk | Cl- ions √1mk Confirmed |
| White ppt√½ mk Insoluble √½ mk | SO42- ions √1mk |
| Pale green solution turns to yellow solution √1mk Brown ppt insoluble in excess √1mk | Fe2+ oxidized to Fe3+ ions √½ mk Fe3+ ions confirmed √½ mk |


